3759
The inhomogeneous MT (ihMT) technique: Achievements and perspectives1Radiology, Division of MR Research, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, United States, 2Aix Marseille Univ, CNRS, CRMBM, Marseille, France
Synopsis
The inhomogeneous (ihMT) technique represents a relatively simple addition to MT, but provides a different contrast that is sensitive to the dipolar relaxation time T1D. A review is provided of: method(s) for its application; considerations for optimizing the ihMT signal; the use of models to obtain quantitative parameters and guide acquisition; and its application in vivo.
Purpose
To provide a review of this relatively new but simply implemented technique, which is based on MT. The review will educate on: the various ihMT acquisition methods; application in studies; optimization of ihMT; and, analysis of the signal including modeling to obtain quantitative parameters.Outline of Content
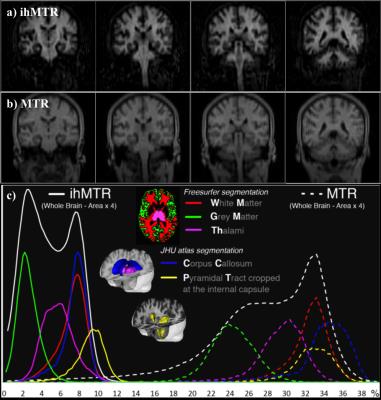
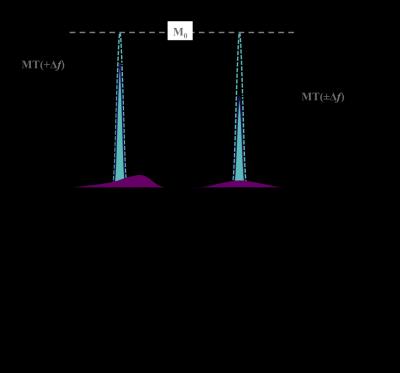
The relatively novel inhomogeneous MT (ihMT) technique has sparked interest based on demonstrations of greater signal in myelin containing tissues, as found in the central nervous system (CNS) (Fig. 1), and close to zero signal from other tissue types1,2. The method is based on the difference in the signal following RF irradiation at a single offset frequency Δf, and the signal from a dual frequency (±Δf) saturation preparation, achieved by cosine modulation or alternating frequency RF pulses (Fig. 2). The potential contribution from MT asymmetry, due to use of data acquired following RF saturation applied at varying offset frequencies, has been studied at ultra-high field3. As for MT, it is possible to optimize the ihMT signal based on the RF saturation scheme4, and can be applied in various means to 2D/3D MRI sequences alike1,2,5.
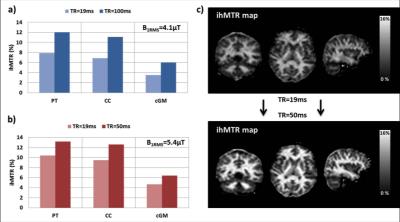
The nomenclature stems from initial thoughts that the differences in saturation are due to inhomogeneous broadening underlying the short T2 line associated with the macromolecular, bound component. In particular, the differential saturation was thought to be more specific to the lipid membranes6,7, found in high concentration in myelin of the CNS, which itself forms a major part of white matter. Greater understanding of the mechanism, including use of a dipolar reservoir in MT models, shows ihMT to be sensitive to the dipolar relaxation time, T1D8 and the result of inhomogeneous saturation behavior of dipolar broadened lines. Single offset frequency saturation effectively couples the Zeeman pool with the dipolar reservoir (assuming a non-zero T1D), thus limiting the efficiency of the saturation. On the other hand, dual-offset irradiation decouples the Zeeman pool from the dipolar order, which gives a more efficient saturation9,10 (Fig. 2a). The larger ihMT signal in the CNS, and lamellar liquid crystalline samples, is associated with spin systems with residual dipolar couplings and relatively long T1D (>3ms), which is itself found to be temperature dependent11. IhMT signal in short T1D tissues, e.g. muscle, are found to be weak but still measurable. The potential sensitivity to white matter and grey matter tissues is improved by optimization of the RF pulses utilized12, and greatly enhanced by low duty RF power deposition13,14 (Fig. 3).
The ihMT technique has been compared with diffusion tensor imaging in healthy spinal cords and shows variation with cervical level, age, and between white matter fascicles15. To date, application of ihMT in studies of: amyotrophic lateral sclerosis16, cervical spondylotic myelopathy17, multiple sclerosis18,19, and a mouse model for experimental autoimmune encephalomyelitis20, has shown ihMT to be a useful complimentary MRI technique. In most cases any changes detected were more significant relative to regular MT.
Summary
The ihMT technique gained attention from initial CNS images displaying a signal sensitive to myelin containing tissues, and little signal from other tissues. Models based on existing MT show ihMT to be sensitive to the T1D parameter. Modification to the ihMT acquisition allows optimization of the ihMT signal, in particular the contribution from shorter T1D components. Preliminary application in studies of neurological disease shows promise.Acknowledgements
No acknowledgement found.References
1. Varma G, et al. Magnetization transfer from inhomogeneously broadened lines: A potential marker for myelin. Magn Reson Med. 2015;73:614–622.
2. Girard OM, et al. Magnetization transfer from inhomogeneously broadened lines (ihMT): Improved imaging strategy for spinal cord applications. Magn Reson Med. 2016;doi:10.1002/mrm.26134.
3. Prevost VH, et al. Minimizing the effects of magnetization transfer asymmetry on inhomogeneous magnetization transfer (ihMT) at ultra-high magnetic field (11.75 T). Magn Reson Mater Physics Biol Med. 2016;29:699–709.
4. Girard OM, et al. Magnetization transfer from inhomogeneously broadened lines (ihMT): Experimental optimization of saturation parameters for human brain imaging at 1.5 Tesla. Magn Reson Med. 2015;73:2111–2121.
5. Varma G, et al. 3D Acquisition of the Inhomogeneous Magnetization Transfer Effect for Greater White Matter Contrast. Proc ISMRM 2013:4224.
6. Maricq MM, et al. NMR in rotating solids. J Chem Phys. 1979;70:3300–3316.
7. Chen JH, et al. High-resolution MAS NMR spectroscopy detection of the spin magnetization exchange by cross-relaxation and chemical exchange in intact cell lines and human tissue specimens. Magn Reson Med. 2006;55:1246–1256.
8. Varma G, et al. Interpretation of magnetization transfer from inhomogeneously broadened lines (ihMT) in tissues as a dipolar order effect within motion restricted molecules. J Magn Reson. 2015;260:67–76.
9. Goldman M. Spin temperature and nuclear magnetic resonance in solids. 1970.
10. Lee JS, et al. Uniform saturation of a strongly coupled spin system by two-frequency irradiation. J Chem Phys. 2011;134:234504.
11. Swanson SD, et al. Molecular, dynamic, and structural origin of inhomogeneous magnetization transfer in lipid membranes. Magn Reson Med. 2016;doi:10.1002/mrm.26210.
12. Duhamel G, et al. T1D-w ihMT: Dipolar relaxation time weighted imaging using inhomogeneous magnetization transfer. in Proc ISMRM 2016:2909.
13. Varma G, et al. Increasing the Inhomogeneous Magnetization Transfer (ihMT) Signal in Vivo with High Amplitude, Low Duty Cycle Irradiation. in Proc ISMRM 2016:2890.
14. Girard O, et al. Theoretical and experimental optimization of a 3D steady-state inhomogeneous (ihMT) gradient echo sequence: Boosting the ihMT sensitivity with sparse energy deposition. in Proc ISMRM 2016:2892.
15. Taso M, et al. Tract-specific and age-related variations of the spinal cord microstructure: a multi-parametric MRI study using diffusion tensor imaging (DTI) and inhomogeneous magnetization transfer (ihMT). NMR Biomed. 2016;29:817–832.
16. Rasoanandrianina H, et al. Regional and Structural Changes of the Spinal Cord Tissue Encountered in Amyotrophic Lateral Sclerosis (ALS): A Preliminary and Promising Characterization using DTI and ihMT. in Proc ISMRM 2016:1500.
17. Taso M, et al. Combining Biomechanical Finite Element Analysis and Multi-Parametric MRI to Assess Mechanical and Structural Damage in Cervical Spondylotic Myelopathy. in Proc ISMRM 2016:847.
18. Varma G, et al. Analysis of Inhomogeneous Mangetization Transfer Applied in Patients with Multiple Sclerosis. in Proc ISMRM 2014:2071.
19. Duhamel G, et al. Magnetization Transfer from Inhomogeneously Broadened Lines (ihMT): Application on Multiple Sclerosis (MS). in Proc ISMRM 2015:4346.
20. Prevost V, et al. Magnetization Transfer from Inhomogeneously Broadened Lines (ihMT): Application on a Mouse Model of Experimental Autoimmune Encephalomyelitis (EAE). in Proc ISMRM 2015:4365.
Figures