3757
Correction of fat artifacts for unbiased CEST-MRI of the human breast at 7 T1Medical Physics in Radiology, German Cancer Research Center, Heidelberg, Germany, 2Division of Radiology, German Cancer Research Center, Heidelberg, Germany
Synopsis
Chemical Exchange Saturation Transfer (CEST) MRI in the mammary gland is affected by the high fat content in the human breast. Chemical shift induced artifacts are visible in the Z-Spectrum. We showed a method to test and verify water-fat separation techniques in vitro. Transfer of the gained insights to realize water-only CEST-MRI in the human breast is currently under investigation.
Purpose
Chemical Exchange Saturation Transfer (CEST) MRI provides insights and
contrasts of tissue degeneration und can be used to detect tumors, i.e. in
brains.1,2
A development of CEST-MRI contrasts towards other organs and body parts is
desired. Adipose fat surrounding tissue and organs, e.g. the mammary gland or
the prostate, not only causes the chemical shift induced fat artifacts, but
also show a significant negative effect within the Z-spectrum in CEST-MRI, so
called pseudo relayed Nuclear Overhauser Effects (rNOEs).3
Therefore, CEST-MRI under these conditions is critical without
consideration of the fat signal. Hence, frequency selective fat suppression or
water-fat separation is crucial and needs to be verified. Here we present a
Dixon-based water-fat separation for the development of CEST-MRI in the human
breast at 7T and a method for validation of its correction of the pseudo rNOE
artifact.Materials and Methods
The chemical shift imaging artifact can be used to shift the fat (F) and water (W) signal in the image by the displacement: $$\Delta s = \Delta\omega_{FW} \cdot \gamma B_0/BW_{pix} \quad \quad \quad \quad \quad (1)$$ with frequency offset ΔωFW ≈ 3.3 ppm between F and W, and the bandwidth per pixel BWpix. The Dixon method4 exploits the chemical shift between F and W for separate imaging of the two signal components. Separation is performed by calculation of images (I) acquired at echo times TE where F and W is in-phase (ip) and in opposed-phase (op):
$$F = 1/2 \cdot (I_{ip} - I_{op}) \quad \quad \quad \quad (2)$$
$$W= 1/2 \cdot (I_{ip} + I_{op}) \quad \quad \quad \quad (3)$$
Water-only Z-spectra were calculated by water-only images according to equation (3).
Measurements were performed on a 7 Tesla whole-body MR tomograph (MAGNETOM 7T, Siemens Healthineers, Germany) using a commercial 1Tx/16Rx bilateral diagnostic breast array for 7T MRI (Rapid Biomedical GmbH, Rimpar, Germany). Presaturation was achieved using 150 Gaussian-shaped pulses (15 ms length, duty cycle DC = 50%) with a mean amplitude of B1 = 0.6µT. Z-spectra were corrected for B0 inhomogeneities. Images were acquired using a 2D-GRE sequence with a readout bandwidth BWpix =200 or 2000 Hz/pix.
A model solution containing raw crude egg was prepared. In vivo CEST data of the human breast were acquired from two healthy volunteers.
Results
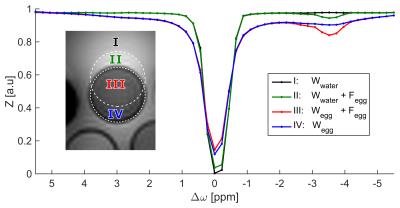
Using a BWpix = 200 Hz/pix was sufficient to induce a
chemical shift displacement strong enough to define four regions of interest
(ROI) with different contributions of the F and W signal (Fig. 1). ROI IV,
measured within fatty tissue but without a fat signal contribution allows
detection of the pure water signal of the egg sample. Consequently, fat artifact
free CEST-MRI is feasible in this region. In contrast, a resonance of the pseudo rNOE was resolved in the Z-spectra of regions with fat signal contribution.
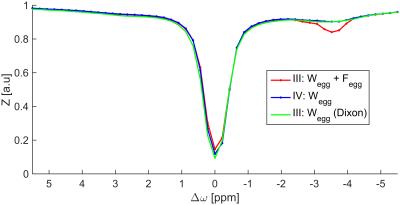
The water-only Z-spectrum achieved by the Dixon method coincides very well with
the fat artifact free Z-spectrum, ROI IV (Fig. 2).
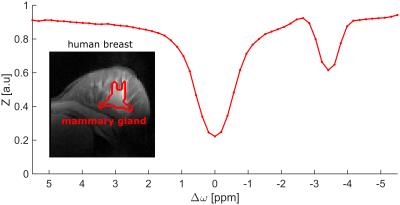
The pseudo rNOE contribution was also prominent in Z-spectra in vivo obtained in the mammary gland of
a healthy volunteer (Fig. 3). However, application of the Dixon method similar
to that of the in vitro experiments did
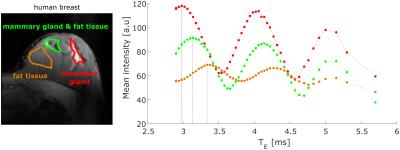
not yield clearly separated water and fat images. In order to optimize the
Dixon method, TE was varied over a broad range of values. Signal
intensities within breast tissue oscillate as expected as a function of TE.
However, positions of maxima (F and W in phase) and minima (F and W in opposed phase)
vary among the breast (Fig. 4).Discussion
Prominent pseudo rNOE artifacts are present in Z-spectra of the human breast, which prevent simple application of conventional CEST-MRI. We showed that the Dixon technique applied to CEST-MRI is feasible to correct for this artifact (Fig. 2). This was verified with the proposed method presented in this study. However, in vivo a dispersion of the frequency offset between water and fat ΔωFW (caused by e.g. B0-inhomogeneities, or the occurrence of multiple fat signals at different resonance frequencies) was observed (Fig.4). Consequently, a more sophisticated Dixon approach5,6 accounting for these issues has to be used for CEST-MRI in the human breast. The data presented is currently used to implement and test such advanced methods.Conclusion
We verified the functionality of the Dixon water-fat separation for unbiased CEST-MRI in fatty samples. However, more advanced techniques are required for water-only CEST-MRI of the human breast. The implementation of a 3pt-Dixon method based on the presented data is now an ongoing process. This will enable robust correction of pseudo rNOE artifacts in the Z-spectrum and thus will allow fat corrected CEST-MRI at 7T in vivo.Acknowledgements
References
1. Zaiss M, Windschuh J, Paech D, et al. Relaxation-compensated CEST-MRI of the human brain at 7T: Unbiased insight into NOE and amide signal changes in human glioblastoma. NeuroImage 2015;112:180–188.
2. Jones CK, Huang A, Xu J, et al. Nuclear Overhauser enhancement (NOE) imaging in the human brain at 7 T. Neuroimage 2013;77:114-124.
3. Lu J, Zhou J, Cai C, et al. Observation of true and pseudo NOE signals using CEST-MRI and CEST-MRS sequences with and without lipid suppression. Magnetic Resonance in Medicine 2015;73(4):1615–1622.
4. Dixon WT. Simple proton spectroscopic imaging. Radiology 1984;153(1):189-194.
5. Wang Y, Li D, Haacke EM, et al. A three-point Dixon method for water and fat separation using 2D and 3D gradient-echo techniques. Journal of Magnetic Resonance Imaging 1998;8(3),703–710.
6.
Yu H, Shimakawa A, McKenzie
CA, et al. Multiecho water-fat
separation and simultaneous R2* estimation with multifrequency fat spectrum
modeling. Magnetic Resonance in Medicine 2008;60(5):1122–1134.
Figures