3753
Chemical exchange rotation transfer (CERT) of human brain at 3 T1Vanderbilt University Institute of Imaging Science, Nashville, TN, United States
Synopsis
It has been shown that the changes in chemical exchange saturation transfer (CEST), and specifically amide proton transfer (APT) and nuclear Overhauser effect (NOE), reflect abnormal tissues in tumor, stroke and other diseases. However, quantitative and specific imaging of these effects is challenging due to the influences from asymmetric magnetization transfer and direct water saturation. These obstacles can be avoided with chemical exchange rotation transfer (CERT), which is a pulsed version of CEST with the constraint of constant average power and varying rotation angle. In this study, we present initial CERT results in human brain at 3 T, with the goal of quantifying APT and NOE.
Purpose
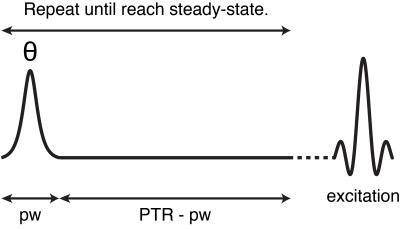
CEST detects endogenous metabolites via proton exchange and nuclear Overhauser effects, and has become an important quantitative tool. It has been shown that the changes in APT reflect the physiological state. However, it is difficult to quantify APT by the conventional metric, MTRasym, which is affected by NOE and asymmetric magnetization transfer (MT). Chemical exchange rotation transfer (CERT)1 is a pulsed version of CEST subjected to the constraint of constant average power (Fig. 1), and CERT can quantify APT and NOE without any model assumption about the signal dependence on irradiation frequency. With this benefit, CERT only requires the measurements at the relevant offsets, which may make CERT-based experiments more practical clinically.Methods
All the images were acquired on a 3 T Achieva Philips MR scanner with 32-channel receive head coil. CERT experiments were performed using a 2 s saturation pulse train with an average power varied between 0.6 μT and 0.9 μT. The pulse width was 12 ms, followed by a 2 ms, 2 mT/m spoiler. The images were acquired with spin echo single-shot EPI (TR/TE = 3000 ms/35 ms) and with a resolution of 2.5 mm x 2.5 mm x 5 mm. APT and NOE are quantified using the MTRdouble metric which is the difference in the water signals when applying π (labeling scan) and 2π pulses (reference scan) at the solute resonance: $$$ MTR_{double}=(S(π)-S(2π))/S_0$$$.Results
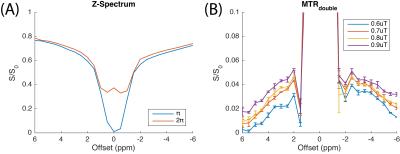
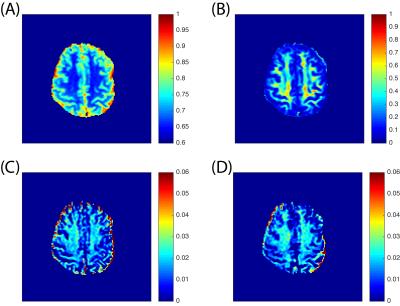
A representative Z-spectrum from 20 voxels in the white matter (WM) region is shown in Fig. 2A. Both labeling and reference scans merge at +/- 6 ppm, showing that the same amounts of power are delivered, which supports the assumption of CERT. However, there are not clear APT and NOE contributions to the MTRdouble metric in out initial results, in contrast to our previous animal results. For the reference scan, the on-resonance signal is higher than the surrounding ones, indicating that the rotation is correctly performed by the train of 2π pulses. MTRdouble (Fig. 2B) shows that APT and NOE in WM region are around 3.5 and -3.5 ppm, respectively, and the strong peaks in the region from -2 to 2 ppm are likely due to the direct water rotations by π and 2π pulses. APT and NOE have different dependencies on the average power such that APT increases from 2.8% to 3.8% and NOE increases from 3.4% to 4.4%, when the average power raises from 0.6 μT to 0.9 μT. The APT (Fig. 3C) and NOE (Fig. 3D) images of MTRdouble are dominated by white matter and gray matter contrasts, and therefore are highly correlated with fractional anisotropy (FA) map (Fig. 3B).Discussion
In order to quantify APT and NOE and to avoid the confounding signal contributions to the MTRasym metric, Lorentzian fitting of the Z-spectrum has been proposed to remove direct water saturation2, and recently, a two-pool MT model was also introduced to remove both direct water saturation and MT3. These methods require several extra scans to obtain reasonable fittings and hence increase the total acquisition time dramatically. CERT is a simple approach to quantify APT and NOE via solute rotations and the MTRdouble metric, and MT background and water saturation can be cancelled when the saturation power is held constant. Ideally, MTRdouble only requires three acquisitions. The MTRdouble APT and NOE peaks correlate with WM tissue in healthy subjects, and we expect to observe contrast between normal and tumor tissues due to changes in the microenvironment and metabolite concentrations. The range of the artifacts near 0 ppm is strongly dependent on the excitation bandwidth of the pulse widths during saturation and the average power. We found that the largest MTRdouble value was at an average power of 0.9 μT on the 3 T human scanner.Conclusion
We show preliminary CERT results on human brain at 3 T. CERT is a model-free method, and moreover, in the ideal case, CERT only requires three scans (normalizer, reference, and labeling), which dramatically reduces the experimental time, and may make CEST more practical clinically.Acknowledgements
No acknowledgement found.References
1. Zu Z, Janve VA, Xu J, Does MD, Gore JC, Gochberg DF. A new method for detecting exchanging amide protons using chemical exchange rotation transfer. Magn Reson in Med 2013;69(3):637-647.
2. Jones CK, Polders D, Hua J, Zhu H, Hoogduin HJ, Zhou J, Luijten P, van Zijl PC. In vivo three-dimensional whole-brain pulsed steady-state chemical exchange saturation transfer at 7 T. Magn Reson Med 2012;67(6):1579-1589.
3. Heo HY, Zhang Y, Jiang S, Lee DH, Zhou J. Quantitative assessment of amide proton transfer (APT) and nuclear overhauser enhancement (NOE) imaging with extrapolated semisolid magnetization transfer reference (EMR) signals: II. Comparison of three EMR models and application to human brain glioma at 3 Tesla. Magn Reson Med 2016;75(4):1630-1639.
Figures