3742
Assessment of hepatic glycogen metabolism ex vivo and in vivo in mice using chemical exchange saturation transfer MRI1Translational Imaging Biomarkers, Merck & Co., Inc, West Point, PA, United States, 2VUIIS, Vanderbilt University, Nashville, TN, United States, 3Vanderbilt Medical Center, Vanderbilt University, Nashville, TN, United States
Synopsis
Despite being integral to whole body glucose homeostasis, liver glycogen remains difficult to measure non-invasively. Recent works have demonstrated the feasibility of detecting liver glycogen using CEST MRI. In this presentation we present data that builds upon this observation and investigate whether CEST can be used to monitor glycogen synthesis and breakdown in mice in real time both ex vivo in a perfused liver system, and in vivo. Treatment with hyperglycemia or glucagon resulted in increases or decreases, respectively, in the CEST MTRasym AUC over time. This demonstrates that CEST-based approaches can be used to non-invasively monitor glycogen metabolism.
Purpose
Glycogen is the primary tissue storage form of carbohydrates and plays in integral role in whole body glucose homeostasis, yet remains difficult to measure without invasive biopsies or sparsely available in vivo 13C magnetic resonance spectroscopy (MRS) hardware. Recent work has demonstrated that glycogen can be detected using chemical exchange saturation transfer (CEST)1. The purpose of this work was to develop a non-invasive, 1H-based approach to measure hepatic glycogen metabolism using CEST in perfused mouse livers at 11.7 T, and in mice in vivo at 7 T. Additionally, we investigated if it was possible to detect increases and decreases in hepatic glycogen metabolism, induced by infusion of glucose/fructose and glucagon, respectively, using CEST.Methods
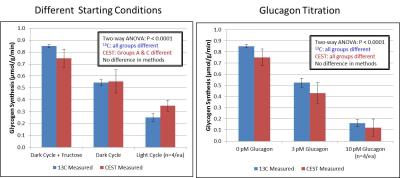
Perfused liver experiments2 were performed on a Bruker 11.7T (500 MHz) wide bore NMR spectrometer using a 20 mm TXO probe tuned for 13C and 1H observation. Approximately 6 month old, non-fasted C57/Bl6 mice were anesthetized during the dark phase of the light/dark cycle and, following a portal vein cannulation, livers were excised and placed in a custom 20 mm NMR tube. Livers were perfused for approximately 3 hrs. with 15 mM [1-13C] glucose alone, or in combination with 1 mM fructose (to increase glycogen synthesis) or varying doses of glucagon (to decrease glycogen synthesis). Glycogen content was monitored in real time using interleaved CEST and 13C MRS acquisitions.
In vivo studies were performed on a Bruker 7 T horizontal bore MRI with a 30 mm volume coil. Mice expressing the human glucagon receptor were used as we have found them to have elevated glycogen levels and elevated rates of glycogen synthesis. Mice were anesthetized with isoflurane and subjected to one of the following protocols: (1) IV [1-13C] Glucose infusion of 1 g/kg over 10 min. followed by 10 mg/kg/min for 100 min. (2) IV glucagon bolus of 30 μg/kg followed by saline infusion, (3) no infusion. CEST MRI Z-images were acquired using a cest_RARE pulse sequence with the following acquisition parameters: 64x64 pixels, RARE factor = 32 (2-shots), centric encoding, saturation pulse = 4 μT, 500 ms, TR / TE = 10 sec / 5 msec, acquisition time = 20 min. Offset frequencies ranges were non-uniformly spaced and ranged from +9 ppm to -9 ppm with tight sampling from +2.5 ppm to -2.5 ppm. Z-spectra and Z-images were processed using in house software written in Matlab using Lorentzian modeling of the Z-spectrum to calculate the MTRasym curve and/or the 0 ppm shift. For the in vivo MRI studies, the slope of the MTRasym AUC vs. time for the serially acquired Z-images (usually 4-5) in each study was calculated on a pixel by pixel basis.
Results/Discussion
Figure 1 shows glycogen synthesis rates from perfused livers separately calculated from interleaved CEST and 13C MRS acquisitions. Rates of glycogen synthesis were found to be elevated when fructose was added to the perfusate and conversely were reduced in a dose-dependent fashion when glucagon was added to the perfusate. Glycogen synthesis rates estimated by both techniques were in good agreement (R2 = 0.65), with the CEST measurement having larger variance overall compared to the 13C MRS measurement.
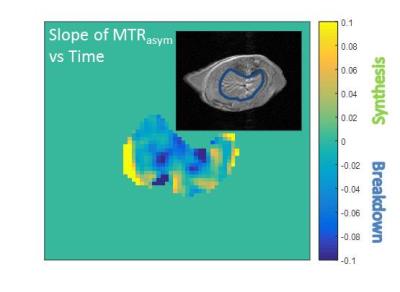
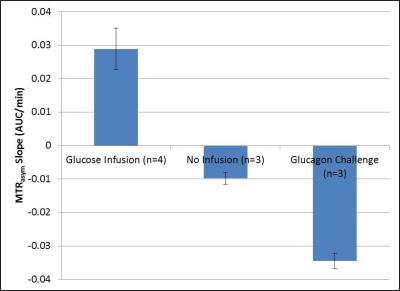
Figure 2 shows sample anatomical and CEST images from a mouse subjected to a glucagon infusion in vivo. This image was formed by calculating the average slope of the MTRasym AUC vs. time and represents a net glycogen breakdown (blue) for most of the liver for this subject. Figure 3 shows average slopes of MTRasym AUC vs. time from ROIs drawn from similar images from mice subjected to the protocols described above. As expected, mice subjected to high glucose infusion had a net increase in glycogen over time while mice subjected to a glucagon challenge had a net decrease. Mice not subjected to an infusion had a minor decrease in glycogen, most likely indicative of normal glycogen breakdown during fasting.
Conclusion
We have implemented CEST-based MR spectroscopic and imaging protocols in isolated mouse livers and in vivo to detect changes in hepatic glycogen content over time. Changes in the CEST MTRasym signal over time agreed with the expected changes in liver glycogen during a glucose infusion (increase) and a glucagon challenge (decrease). This work demonstrates that CEST-based MR methods can be used to detect hepatic glycogen metabolism.Acknowledgements
No acknowledgement found.References
1. Van Zijl, P. C. M. Jones, C. K. Ren, J. Malloy, C. R. Sherry, A. D. PNAS. 2012, 104(11), 4359-4364.
2. Cohen, S.M. In Research in Perfused Liver: Clinical and Basic Applications. Ballet, F. and Thurman, RG. Eds. John Libbey: London, 1991, Chapter 4.
Figures