3734
Kitchen Safe T1 and T2 Phantom Creation1HeartVista, Los Altos, CA, United States
Synopsis
Typical magnetization prepared phantoms require paramagnetic salts such as copper, nickel and manganese chloride, which are toxic. Accordingly, governing bodies label these as hazardous materials requiring special storage, labeling and disposal. In search of a simpler and cheaper solution we have revisited this topic to make a kitchen-safe phantom recipe that is both cheap and easy to make. We used glycerine (a sweetener or for skin care) and Agar agar powder (a food additive for thickening or gel) to make phantoms with a physiological range of T1 and T2, and characterize these non-hazardous materials for 1.5 T MRI scanners.
Introduction
Typical magnetization prepared phantoms require paramagnetic salts such as copper chloride, copper sulfate, nickel chloride and manganese chloride, which are a skin irritants due to human toxicity. Accordingly, various governing bodies label these agents as hazardous materials requiring special storage, labeling and disposal. Several non-hazardous materials have been used in phantom creation, but their properties have not been characterized for clinical MRI scanners of field strengths of 1.5 and 3 T [1], [2]. In search of a simpler and cheaper solution we have revisited this topic to make a kitchen-safe phantom recipe that is both cheap and easy to make.Methods
Samples were created using distilled water, glycerine and agar agar powder acquired from consumer stores. A 10 mg precision scale was used for all measurements. Because the melting point of agar agar gel is approximately 85 C, the solutions were repeatedly brought to a boil in a microwave and mixed until a clear homogeneous mixture was apparent (up to triple boiling). Any loss of mass from heating and mixing was replaced by hot water and mixed. Sample mixtures were then poured in 1 oz Nalgene bottles (35 mm diameter), fitting approximately 40 ml of fluid, and labeled. Bottles were surrounded by water during imaging to reduce susceptibility artifacts.
Twenty six samples were created with varying glycerine and agar concentrations and analyzed along other similar phantoms. Glycerine concentration varied between 0% and 50% while agar concentration varied from 0% to 20%. Imaging was carried out on a 1.5 T GE twinspeed MRI scanner. Some mixtures were created as exploratory and others to target physiological tissue magnetization properties.
T1 measurements were completed by modeling T1 recovery of spin echo inversion recovery images. The TR was set to 4000 ms and the TE varied from 20ms to 2000ms. The model accounted for incomplete inversions and incomplete T1 recovery because the TR was set to less than 10x the expected T1 values.
T2 measurements were completed by modeling T2 decay of a series of spin echo images of 1.25 mm resolution and 8 mm slice thickness. The imaging was completed with a 35 degree flip angle and a TR of 2000 ms and TEs of 14, 20, 30, 50, 70, 100, 250, 500, 1000 and 1500 ms.Estimates of T1 and T2 relaxivity were completed using regression analysis with respect to concentration of magnetic agent. Regression analysis was used to find linear or quadratic trends of relaxation with respect to concentration of agar and glycerine.
Results
Fig 1 provides a view of the multiple phantom setup. Samples provided a range of T1 species from 3024 ms (pure water) to 298 ms (50% glycerine and 15% agar). Regression analysis of samples yielded linear and quadratic trends in relaxivity with respect to concentration agar and glycerine. For concentration percentage of agar and glycerine, A and G respectively, the following relationships were found: Agar agar T1 relaxivity was linear as A* 0.068 (1 / sec). Agar agar T2 relaxivity was linear as A * 7.775 (1 / sec). Glycerine T1 relaxivity was quadratic and fit the regression of G*5.33E-4 + G*G*3.26E-4 (1/sec). Glycerine T2 relaxivity was linear as G * 0.061 (1 / sec).
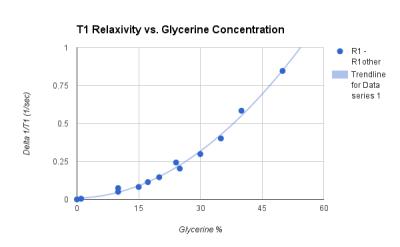
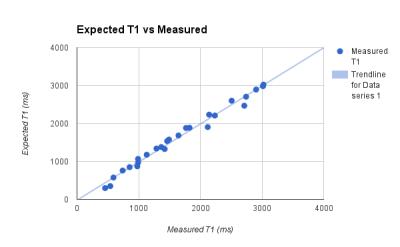
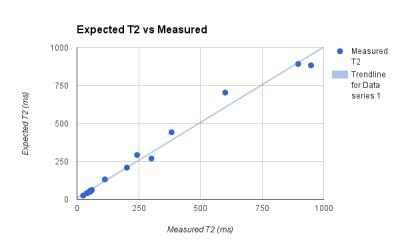
Once the T1 relaxation of both water and agar are removed, Fig. 2 illustrates a clear quadratic relationship between glycerine concentration and T1 relaxation. Fig 3 and Fig 4 illustrate the correlation of the regression analysis on concentrations and the actual relaxation parameters of the various samples. Over 6 months, stability and bacterial growth have not seemed to be an issue for any of the sealed samples.
Conclusion
We have revisited the topic of phantom creation to target T1 and T2 species in search of a simpler and cheaper alternatives to hazardous materials and characterized these materials for 1.5 T MRI systems. The choice of the food agar agar provides good T2 and T1 shortening, while household glycerine provides good T1 shortening. Both agents provide predictable magnetization properties for targeting physiologic range.Acknowledgements
No acknowledgement found.References
[1] Bucciolini M, Ciraolo L, Lehmann B. Simulation of biologic tissues by using agar gels at magnetic resonance imaging. Acta Radiologica, vol 30, 667-669. 1987.
[2] Blechinger J. Tissue-mimicking gelatin-agar gels for use in magnetic resonance imaging phantoms. Medical Physics, vol 15, 629. 1988.
Figures