3731
Novel nanogel-based MRI Contrast Agents1Department of Chemistry, University of York, YORK, United Kingdom, 2Centre for Hyperpolarisation in Magnetic Resonance, University of York, YORK, United Kingdom
Synopsis
We present here the preparation and properties of new Gd3+-containing nanogel-based contrast agents. These gel nanoparticles are prepared by simple ionotropic gelation from sodium alginate and Gd3+. The nanogels are 100-200 nm in diameter and have a high R1 relaxivity of ≈ 30 mM-1 s-1 at 1 T, however, they have poor stability in high ionic strength media. Adding further covalent crosslinks with a suitable agent such as epichlorohydrin dramatically increased the relaxivity of the nanogels to ≈ 60 mM-1 s-1 at 1 T and provides a viable strategy to increases their stability against transmetallation.
Purpose
The project aims to explore the potential of self-assembled, nanoparticulate, biocompatible, polysaccharide polymer gels containing Gd3+ ions to act as a high relaxivity contrast agent platform.Background
At the magnetic field strengths employed in current clinical MRI machines the molecular tumbling rate of contrast agents is generally the limiting factor in their efficiency (relaxivity). Strategies to deliver high loads of Gd3+ ions with slow local tumbling have concentrated on appending Gd3+ chelates to macromolecular systems. Generally these approaches provide only modest gains in relaxivity and would not be practical commercially due to scaling or safety considerations. An area that has received comparatively less attention until very recently is the incorporation of contrast agents into nano-sized hydrogel (nanogel) systems.1-4 Hydrogels are three-dimensional hydrophilic polymer networks that can absorb and retain large quantities of water, saline or physiological solutions. Using a nanogel approach has several advantages; nanogels are stable and have well defined physical and chemical characteristics in aqueous media, they can be highly loaded with molecules of interest, provide fast water diffusion and highly accessible but immobilised metal centres. Because of these favourable properties nanogel systems are seeing increased attention in the fields of drug delivery and theranostics.Results and Discussion
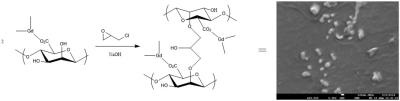
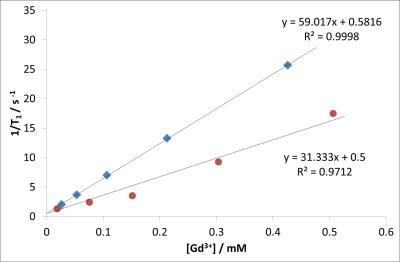
Several naturally occurring, biocompatible polysaccharide polymers spontaneously undergo a gelling process in the presence of metal ions (ionotropic gelation). Alginates are the most widely used and due to their low-toxicity, low cost and relative ease of functionalisation they have received intense investigation for use in biomedical applications such as wound healing, tissue engineering and drug delivery.5 Nanogels containing Gd3+ ions can be prepared simply by ionotropic gelation of sodium alginate with Gd3+ solutions via nebulisation 6 or high dilution 7 methods in a similar manner to Ca2+ or Zn2+ crosslinked particles (Fig. 1). These nanogels were found to be stable in millipore water and to have a size of 100-200 nm which was confirmed by SEM and Dynamic Light Scattering (DLS). Such a size is seen as the ideal for in vivo applications.8 These Gd3+-crosslinked particles show high R1 (and R2) relaxivities and have the potential to provide MRI contrast with an R1 relaxivity at 1 T of ≈ 30 mM-1 s-1.However, crosslinked alginate gels are relatively mobile at a molecular scale 9 and these ionically crosslinked particles show poor stability in the presence of high concentrations of metal ions such as Na+ in saline solution. In order to provide increased structural stability the particles can be further covalently crosslinked by a suitable cross-linking agent e.g. epichlorohydrin. In an idealised, completely crosslinked, situation the gadolinium ions can now be thought of as being chelated by macrocyclic ligand systems providing increased thermodynamic and kinetic stability to transmetallation. The covalent crosslinking also provides a more rigid local environment for the Gd3+ ion and leads to a dramatic increase in R1 relaxivity to ≈ 60 mM-1 s-1 at 1 T. The stability of these crosslinked nanogel particles towards metal leaching was investigated by measuring changes in their relaxivity after introduction into solutions of NaCl, ZnCl2 or PBS buffer (Fig 4). The results indicate an immediate dramatic decrease in relaxivity corresponding to a rapid swelling of the particles in high ionic strength media, supporting experiments show that this relaxivity change is not due to immediate loss of large quantities of Gd3+ from the particles. In NaCl solution there is slow transmetallation before equilibrium is reached. Addition of Zn2+ ions and HPO42- ions show similar behaviour but now the reduction in relaxivity due to initial swelling is greater. Precipitation of insoluble GdPO4 occurs over a period of days/weeks in the presence of HPO42-. Optimisation of the physical/rheological properties of the nanogel particles may reduce these processes to a viable level.Conclusions
We have shown a novel, promising, simple nanogel approach towards achieving extremely high relaxivity contrast agents with a 5-10 fold efficiency increase over commercially available agents. This nanogel approach has further advantages in that other payloads can be incorporated into the particles leading to possible multi-modal imaging or theranostic applications. The responsive and adaptive nature of nanogels also lends itself readily to incorporation of smart functionality. Efforts to optimise the system to provide the highest relaxivity and stability properties by choice of polysaccharide, cross-linking agent and reaction conditions are underway.Acknowledgements
The authors wish to thank the Daphne Jackson Trust for a fellowship to Brendan Garrett as well as the University of York and the Royal Society of Chemistry for funding.References
1. T. Courant et al, Hydrogels incorporating GdDOTA: towards highly efficient dual T1/T2 MRI contrast agents, Angew. Chem. Int. Ed. 2012, 51, 9119
2. A. Soleimani et al, Polymer cross-linking: a nanogel approach to enhancing the relaxivity of MRI contrast agents, J. Mater. Chem. B., 2013, 1, 1027
3. J. Lux et al, Metal chelating crosslinkers form nanogels with high chelation stability, J. Mater. Chem. B., 2013, 1, 6359
4. M. Callweart et al, Tuning the compostition of biocompatible Gd nanohydrogels to achieve hypersensitive dual T1/T2 MRI contrast agents, J. Mater. Chem. B., 2014, 2, 6397
5. K.Y. Lee and D.J.Mooney, Alginate: Properties and biomedical applications, Prog. Polym. Sci, 2012, 37, 106
6. D. Guzman–Villenueva, et al, A Novel Aerosol Method for the Production of Hydrogel Particles, J. Nanomater., 2011, Article ID 507508
7. S. Pistone, D. Qoragllu, G. Smistad, and M. Hiorth, Formulation and preparation of stable cross-linked alginate–zinc nanoparticles in the presence of a monovalent salt, Soft Matter, 2015, 11, 5765
8. Li Tang et al, Investigating the optimal size of anticancer nanomedicine, PNAS, 2014, 111, 15344
9. G. Ionita et al, Ion exchange in alginate gels – dynamic behavior revealed by electron paramagnetic resonance, Soft Matter, 2015, 11, 8968
Figures

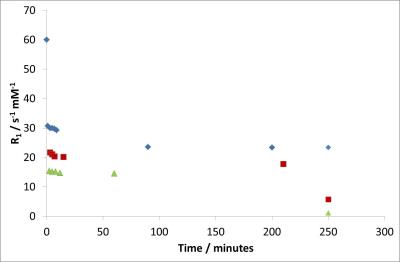
Plots showing how the relaxivity of covalently crosslinked nanogel suspensions (0.25 mM Gd3+) changes after introduction into solutions of 150 mM NaCl (blue diamonds), 0.5 mM ZnCl2 (red squares) and PBS buffer (green triangles). All concentrations are final values. Note all values at '250 minutes' correspond to a time of three weeks showing giving a measure of longer term stability.