Shengwen Deng1, Eric Muir2, Wei Zhou3, and Timothy Q. Duong2
1University of Texas Health Science Center at San Antonio, San Antonio, TX, United States, 2University of Texas Health Science Center at San Antonio, 3Radiology, Mayo Clinic
Synopsis
T1 mapping is showing great
potential for mapping oxygen in human organs such as eyes and lungs. And yet,
accuracy of oxygen using fast T1 imaging methods is of great concern especially
in tissue with radical temperature changes. In the current study we improve fast
T1 mapping with temperature correction and explore its potential in mapping
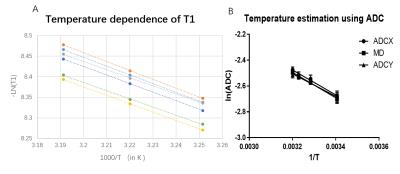
oxygen in eyes. With combination of inversion-recovery Look-Locker bSSFP and diffusion weighted thermometry, we calibrate the temperature dependence of ADC and T1, and use
it to adjust the R1 for measuring partial pressure of oxygen(pO2). Fast T1
mapping could be a reliable way to pO2 that well agrees with invasive oxygen-sensitive
optic fibers.
Acknowledgements
Eric
Muir, Ph.D holds a Voelcker Fund Young Investigator Award from the Max and
Minnie Tomerlin Voelcker Fund.References
Reference:
[1] Muir E R, Zhang Y, San
Emeterio Nateras O, et al. Human vitreous: MR imaging of oxygen partial
pressure[J]. Radiology, 2013, 266(3): 905-911.
[2] Mills R. Self-diffusion in
normal and heavy water in the range 1-45. deg[J]. The Journal of Physical
Chemistry, 1973, 77(5): 685-688.
[3] Hindman J C, Svirmickas A,
Wood M. Relaxation processes in water. A study of the proton spin-lattice
relaxation time[J]. The Journal of Chemical Physics, 1973, 59(3): 1517-1522.
[4] Simpson, Andrew RH, et al.
"Measuring the Effect of Pars Plana Vitrectomy on Vitreous Oxygenation
Using Magnetic Resonance ImagingEffects of PPV on pO2 Using MRI."
Investigative ophthalmology & visual science 54.3 (2013): 2028-2034.
[5] Plata, Juan C., et al.
"A feasibility study on monitoring the evolution of apparent diffusion
coefficient decrease during thermal ablation." Medical physics 42.9
(2015): 5130-5137.