3716
Whole Body T1 Mapping of Small Animals using Prospective Gating and Variable Flip Angle Imaging1CRUK/MRC Oxford Institute for Radiation Oncology, University of Oxford, Oxford, United Kingdom
Synopsis
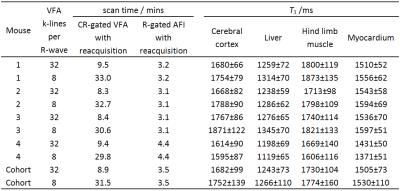
Prospective gating and automatic reacquisition of data corrupted by respiration motion were implemented in variable flip angle (VFA) and actual flip angle imaging (AFI) scans to enable cardio-respiratory synchronised T1 mapping of the whole mouse. T1 calculation for each mouse took approximately 6 s using a robust and efficient nonlinear least squares process. 16 cardio-respiratory gated VFA scans and a respiration gated AFI scan were acquired in less than 14 minutes. T1 was calculated in the whole mouse with a voxel size of 0.075 mm3 and with a standard deviation less than 6.2% within ROIs from multiple organs.
Introduction
The 3D steady state Variable Flip Angle (VFA) method offers the best opportunity for fast and accurate T1 measurement when resolution, sample coverage, and sources of error are considered.1 Cardio-respiratory (CR) ‘spin-conditioned’ gating 2 is required to eliminate motion artefacts from in vivo respiratory and cardiac functions during steady state imaging close to the heart. The VFA method has been implemented in cardiac MR with retrospective CR-gating using an interleaved navigator echo.3 However, retrospective gating is less efficient and offers less motion artefact control than prospective techniques.4 The efficacy of prospective gating for fast and accurate T1 mapping is presented.Methods
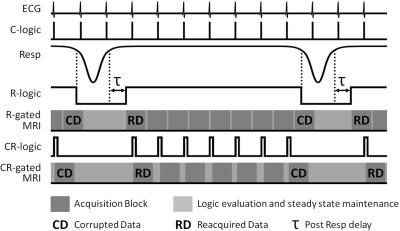
Data were acquired from CBA mice (n=4) on a 7.0 T preclinical MRI system. Anaesthesia was maintained with 1-3% isoflurane in a 1:5 O2:air mixture. Respiration was monitored using a pressure balloon. ECG needles were placed subcutaneously in the chest. An ECG setup free of gradient noise is essential for prospective CR-gating at constant TR with rapidly switched strong magnetic field gradients. A physiological monitoring system was used to generate logic signals for the prospective R-gating and CR-gating strategies presented in Figure 1 which include the automatic and immediate reacquisition of data corrupted by respiration motion. A custom-built unit was used to set ECG logic signals of suitable duration: greater than the time between successive logic signal level reads (ideally the sequence TR); but shorter than the duration of an imaging block acquired in response to the gating signal (typically several TRs). Only the ECG signals that occur during inter breath periods were selected for CR-gating. CR-gated VFA was performed with a 3D RF and gradient spoiled gradient echo and TR 2.8 ms, TE 1.0 ms, FOV 108×27×27 mm3, matrix 256×64×64, hard pulse 16 μs, 16 FAs nominally set from 2° to 8° in steps of 0.4°, centric out phase encoding with 32 k-lines or 8 k-lines per R-wave. 3D B1 maps to quantify flip angle prescription were acquired with a R-gated actual flip angle imaging (AFI)5 scan and TR1 10 ms, TR2 100 ms, TE 0.59 ms, FOV 108×27×27 mm3, matrix 128×32×32, hard pulse 128 μs, nominal FA 64°. T1 calculation was performed with a robust and efficient nonlinear least squares process since results of the widely accepted linear fit are biased and can lead to overestimation of T1 by up to 20%.6Results
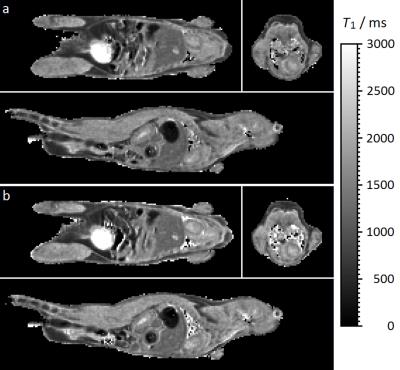
The automatic and immediate reacquisition of data corrupted by respiration motion robustly eliminates respiration motion artefact. Table 1 shows the duration of CR-gated VFA scans and R-gated AFI scans used to generate 3D T1 maps of the 4 mice together with the T1 values of some example tissues. Figure 2 shows example T1 maps through the heart of mouse 1. The nonlinear least squares T1 calculation took approximately 6 s for each mouse. The image data and calculated T1 maps are available courtesy of the Bodleian Digital Library Systems and Services of the University of Oxford at http://dx.doi.org/10.5287/bodleian:a4awG5r7R. Image stability can be inspected by selecting a plane in 3D data sets and scrolling through the time course. Phantom data injected with noise to mimic in vivo stability demonstrated that the VFA-AFI method is able to generate essentially the same results as IR with a SD that was conservatively estimated to be less than 6.2% for T1 values ranging from 400 ms to 2150 ms.Discussion
The T1 values measured from VFA scans with 32 k-lines per R-wave are in good agreement with those measured from scans with 8 k-lines per R-wave that took four times longer, even for myocardium. The duration of VFA scans with 32 k-lines per R-wave was 8.9 mins on average whereas with 8 k-lines per R-wave it was 31.5 mins on average. The results suggest that the scan time benefit of acquiring 32 k-lines per R-wave does not significantly compromise data synchronisation with the cardiac R-wave. Furthermore, it should be noted that the SDs for the cohort are lower in data acquired with 32 k-lines per R-wave. Presumably this is a result of the improved stability that results from the shorter scan time. The volumetric VFA approach generates T1 weighting over a timescale of up to several T1 but maintains it with a timescale of TR which is 2.8 ms in this instance. Since the heartbeat is much slower, synchrony with the cardiac R-wave can always be achieved to within one TR, even in the presence of severe arrhythmia.
Conclusion
Prospective gating and variable flip angle imaging enables fast and accurate cardio-respiratory synchronised T1 mapping of small animals.Acknowledgements
The work presented was supported financially by Cancer Research UK (CRUK grants C5255/A12678, C2522/A10339), the Engineering and Physical Sciences Research Council (EPSRC grant C2522/A10339) and the Medical Research Council Unit Grant for the Oxford Institute for Radiation Oncology. The authors thank John Prentice and Gerald Shortland for mechanical workshop support.References
1. Cheng H-LM, Wright GA. Rapid high-resolution T1 mapping by variable flip angles: Accurate and precise measurements in the presence of radiofrequency field inhomogeneity. Magn Reson Med 2006;55:566-574.
2. Ehman RL, McNamara MT, Pallack M, Hricak H, Higgins CB. Magnetic resonance imaging with respiratory gating: techniques and advantages. Am J Roentgenol 1984;143:1175-1182.
3. Coolen BF, Geelen T, Paulis LEM, Nauerth A, Nicolay K, Strijkers GJ. Three-dimensional T1 mapping of the mouse heart using variable flip angle steady-state MR imaging. NMR Biomed 2011;24:154-162.
4. Bishop J, Feintuch A, Bock NA, Nieman B, Dazai J, Davidson L, Henkelman RM. Retrospective gating for mouse cardiac MRI. Magn Reson Med 2006;55:472-477.
5. Yarnykh VL. Actual flip-angle imaging in the pulsed steady state: a method for rapid three-dimensional mapping of the transmitted radiofrequency field. Magn Reson Med 2007;57:192-200.
6. Chang L-C, Koay CG, Basser PJ, Pierpaoli C. Linear least-squares method for unbiased estimation of T1 from SPGR signals. Magn Reson Med 2008;60:496-501.
Figures