3710
Production of Highly Polarized Acetate by Rapid Decarboxylation of Pyruvate – Application to Hyperpolarized Cardiac Spectroscopy1Institute for Biomedical Engineering, University and ETH Zurich, Zurich, Switzerland
Synopsis
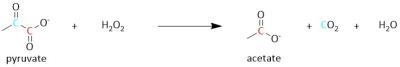
In this work rapid decarboxylation of [1,2-13C]pyruvate using hydrogen peroxide was employed to obtain hyperpolarized [1-13C]acetate. 13C polarization was transferred completely and reproducibly. The application of the concept is demonstrated for detecting [1-13C]acetate and [1-13C]acetylcarnitine in the in-vivo heart.
Introduction
Altered substrate metabolism in the heart precedes
reduced function and clinical symptoms in various diseases.1,2 To this end, probing myocardial utilization of the two main energy sources
fatty acids and glucose is of great interest. Due to its favorable chemical and
physical properties, hyperpolarized [1-13C]pyruvate has been used to probe
glucose metabolism.3–5 Fatty acid metabolism using acetate,
a short chain fatty acid (SCFA), has been explored in the preclinical realm.6 However, acetate is characterized by an inherently low polarization in
comparison to pyruvate7 and hence its applicability remains limited.
The objective of the present work was to
develop and implement an efficient approach to hyperpolarize [1-13C]acetate
and apply it to in-vivo cardiac spectroscopy.Methods
Acetate production
By employing decarboxylation using hydrogen peroxide, [1,2-13C]pyruvate can be split yielding carbon dioxide and [1-13C]acetate (Figure 1). Due to the rapid nature of this reaction, no significant loss of 13C polarization occurs. In addition, by optimizing the pH of the solvent, the reaction can be shifted to achieve a high yield of both or one of the products.8
Hyperpolarization
A home-built multisample dynamic nuclear polarization system9 was used to dynamically polarize samples consisting of 50.8µL [1,2-13C]pyruvic acid and 13.5mM trityl, doped with 1mM Dotarem. After 90 minutes of polarization at 1.3K, the sample was dissolved in 8mL Tris buffer (pH = 12.4). An amount of 62 µL 30% hydrogen peroxide was placed in the collecting vessel for the dissolution medium, ensuring immediate mixing with the [1,2-13C]pyruvate solution. Upon dissolution, the mixture was absorbed into a 5mL syringe and transported to the scanner where it was injected at a rate of 0.1ml/s using a custom built injection system.
Magnetic resonance spectroscopy
Spectroscopic experiments were performed on a 9.4-T MR imaging system (Biospec 94/30; Bruker BioSpin, Ettlingen, Germany). A rectangular 13C surface coil with a sensitive area of 40x30 mm2 (Rapid Biomedical, Wurzburg, Germany) was placed over the thorax for signal reception. A birdcage dual 1H/13C coil (Rapid Biomedical) was used for excitation. In-vivo dynamic 13C spectra were acquired with slice selective spectroscopy using a flip angle of 20° and a repetition time of 1s (slice thickness: 10mm). Acquisitions were triggered to peak systole and end-respiration.
Three female Spargue Dawley rats weighing 330-360g were anesthetized with 4% isoflurane in a mixture of air and oxygen (4:1), endotracheal intubation was performed, and ventilation was initiated. Anesthesia was maintained by using 1-2% isoflurane. Body temperature was kept at 37–38°C by using a warm water heating system. Two 26-gauge intravenous cannulas were placed in opposite sides of the tail, one to allow injection of the dynamic nuclear polarization substrate and one for continuous glucose infusion (15 mg/kg/min).
Data processing
Resonances were fitted using the AMARES10 algorithm as implemented in the jMRUI software package 5.2.11 The [1-13C]acetate resonance was fitted and eliminated12 prior to quantification of [1-13C]acetylcarnitine and [13C]bicarbonate.
Results
Phantom experiments
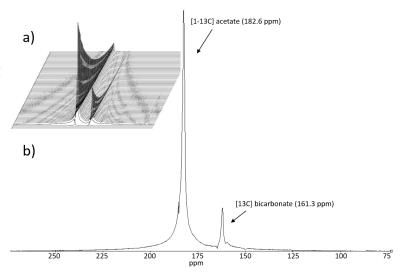
Phantom dissolutions yielded a mean liquid state polarization of 41.0±5.6% for [1-13C]pyruvate and 36.8±6.3% for [1-13C]acetate, extrapolated to the time of dissolution. The fitted T1 of [1-13C]acetate was 44±4.6s. Peaks in the spectrum were identified as [1-13C]acetate and [13C]bicarbonate (Figure 2). No peaks originating from [1,2-13C]pyruvate were observed. Concentrations of no / full conversion were determined using leftover dissolution liquid, and found to be 67+/-6mM (n=8) for pyruvate (without H2O2) and 55+/-0 mM (n=7) for acetate (with H2O2). The injected solution was neutralized by carbon dioxide released from pyruvate (pH=8.2±0.3).
In-vivo experiments
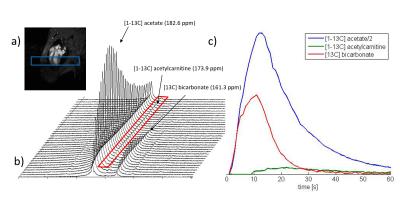
[1-13C]acetate, [1-13C]acetylcarnitine, and [13C]bicarbonate were consistently detected. Signal-time curves were fitted. Exemplary data is shown in Figure 3. The area under the curve of [1-13C]acetylcarnitine (AUCAcCar) normalized by AUCAcetate was 2.5±0.4, 3.5±0.4%, and 4.1±0.2% (mean±standard deviation, 3 repetitions). Peak SNRAcetate was 272±93 and SNRAcCar 8.9±5.3.
Discussion
In the phantom, polarization and
concentration levels of [1-13C]acetate were found to be in the same range as observed
for [1-13C]pyruvate. This indicates that 13C spin-polarization is efficiently
kept throughout the decarboxylation process of pyruvate. In-vivo, [1-13C]acetate and its metabolite [1-13C]acetylcarnitine
could be detected and were analyzed over time repeatedly.Conclusion
These preliminary results demonstrate the feasibility
of employing chemical conversion of pyruvate to acetate in animal experiments
to probe SCFA metabolism. The reaction enables higher polarization than
achievable when polarizing acetate directly.7 Despite the low signal-to-noise ratio of acetylcarnitine, the
results presented hold potential for assessing SCFA metabolism in a robust way.Acknowledgements
The authors acknowledge funding from the Swiss National Science Foundation, grant 320030_153014.References
1. Khairallah, M. et al. Metabolic and signaling alterations in dystrophin-deficient hearts precede overt cardiomyopathy. J. Mol. Cell. Cardiol. 43, 119–129 (2007).
2. Buchanan, J. et al. Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology 146, 5341–5349 (2005).
3. Khemtong, C. et al. Hyperpolarized 13 C NMR detects rapid drug-induced changes in cardiac metabolism. Magn. Reson. Med. 74, 312–319 (2015).
4. Oh-Ici, D. et al. Hyperpolarized Metabolic MR Imaging of Acute Myocardial Changes and Recovery after Ischemia-Reperfusion in a Small-Animal Model. Radiology 278, 742–51 (2016).
5. Schroeder, M. a. et al. Hyperpolarized 13C magnetic resonance reveals early- and late-onset changes to in vivo pyruvate metabolism in the failing heart. Eur. J. Heart Fail. 15, 130–140 (2013).
6. Flori, A. et al. Real-time cardiac metabolism assessed with hyperpolarized [1- 13 C]acetate in a large-animal model. Contrast Media Mol. Imaging 10, 194–202 (2015).
7. Flori, A., Liserani, M., Bowen, S., Ardenkjaer-Larsen, J. H. & Menichetti, L. Dissolution Dynamic Nuclear Polarization of Non-Self-Glassing Agents: Spectroscopy and Relaxation of Hyperpolarized [1-13C]Acetate. J. Phys. Chem. A 119, 1885–1893 (2015).
8. Drachman, N. et al. In vivo pH mapping of injured lungs using hyperpolarized [1-(13) C]pyruvate. Magn. Reson. Med. 1–10 (2016). doi:10.1002/mrm.26473
9. Krajewski, M. et al. A multisample dissolution dynamic nuclear polarization system for serial injections in small animals. Magn. Reson. Med. (2016). doi:10.1002/mrm.26147
10. Vanhamme, L., Van Den Boogaart, A. & Huffel, S. Van. Improved Method for Accurate and Efficient Quantification of MRS Data with Use of Prior Knowledge. J. Magn. Reson. 129, 35–43 (1997).
11. Stefan, D. et al. Quantitation of magnetic resonance spectroscopy signals: the jMRUI software package. Meas. Sci. Technol. 20, 104035- (2009).
12. de Beer, R. et al. Application of time-domain fitting in the quantification ofin vivo1H spectroscopic imaging data sets. NMR Biomed. 5, 171–178 (1992).
Figures