3707
13C-MR Hyperpolarization of Lactate using ParaHydrogen and metabolic transformation in vitro.1Department of Molecular Biotechnology and Health Sciences, University of Torino, Torino, Italy
Synopsis
Hyperpolarization (HP) of the 13C magnetic resonance signal of [1-13C]-lactate has been obtained using the ParaHydrogen Induced Polarization by means of Side Arm Hydrogenation (PHIP-SAH). Different ester derivatives of lactate have been tested in order to optimize the hydrogenation kinetics and the polarization level on the product. The metabolic transformation of hyperpolarized [1-13C]-lactate into pyruvate has been observed in vitro. The bio-compatibility of the aqueous solution and the good polarization level (7.9±0.4%) make the hyperpolarized metabolite thus obtained a good candidate for metabolic imaging studies.
Introduction
The sensitivity of 13C magnetic resonance can be increased by several orders of magnitude thanks to hyperpolarization methods. The application of 13C-MR hyperpolarized molecular probes to in vivo studies had been demonstrated, for the first time, using ParaHydrogen Induced Polarization (PHIP)[1]. The applicability of this easy to handle and cheap method has been hampered, so far, by the fact that a de-hydrogenated precursor of the target molecule is necessary to exploit parahydrogen as hyperpolarization source. On the contrary Dynamic Nuclear Polarization and dissolution (d-DNP) does not suffer from those limitations [2] and it can be applied, in principle, to any substrate. Hyperpolarization of metabolites and, in particular, of pyruvate allowed to investigate metabolic transformations in vivo, in real time. Thanks to their potential clinical role, these metabolic imaging studies quickly evolved from pre-clinical research to clinical trials5.However, the wide application of this powerful tool is limited to few laboratories due to the high costs of instrumentation. The recent introduction of the so called PHIP by means of Side Arm Hydrogenation (PHIP-SAH) [3] allowed to extend the applicability of this method to metabolites, among all to pyruvate, that were not accessible to this hyperpolarization method before. The application of substrates hyperpolarized using PHIP-SAH to metabolic studies needs optimization of the whole procedure and the method can be used to hyperpolarize several metabolites, in addition to pyruvate and acetate. In the herein presented work, [1-13C]-lactate is hyperpolarized by means of PHIP-SAH, some of the limitations of the firstly reported method have been overcome and the first, in vitro, metabolic transformations of these hyperpolarized molecules are observed.Methods
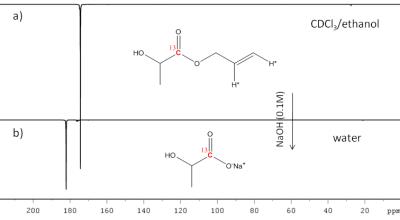
The PHIP-SAH method relies on the following steps: i) functionalization of the molecule of interest (carboxylate containing molecule) with an unsaturated side-arm (an unsaturated alcohol); ii) hydrogenation of the unsaturated moiety using para-enriched hydrogen; iii) polarization transfer from the parahydrogen protons to the target 13C carboxylate signal; iv) (potential) removal of the hydrogenated side-arm by means of hydrolysis. Two different precursors of lactate, vinyl and propargyl esters, have been tested. Hydrogenation reactions have been carried out using 92% enriched parahydrogen and polarization transfer has been made by means of Magnetic Field Cycling. Hydrolysis has been obtained using a diluted Sodium hydroxide solution (0.1M). Phase extraction of the hydrolysed metabolite has been applied [4]. The metabolic transformation of hyperpolarized [1-13C]-lactate into [1-13C]-pyruvate has been tested using a buffered solution of LDH, NAD+/NADH and sodium pyruvate and a cells lysate.Results and discussion
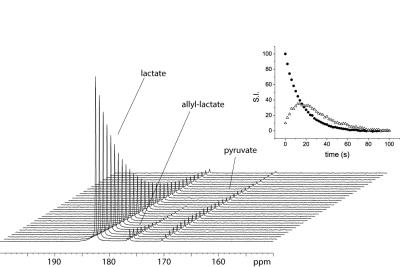
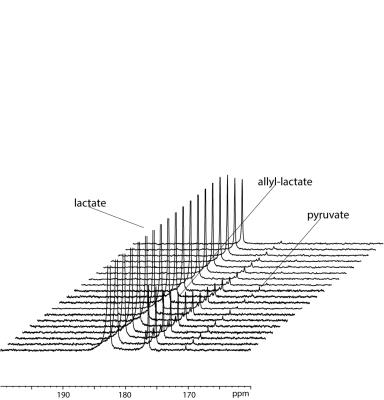
According to the detected polarization (4.5% on the ester) propargyl lactate has been chosen to obtain hyperpolarized [1-13C]-lactate. The phase extraction of this precursor has been performed, the Rh(I) catalyst is retained in the organic phase and an aqueous solution of hyperpolarized metabolite (about 3% polarization, 60mM) has been obtained. Following to the perfusion of the hyperpolarized metabolite to the enzyme solution, the build-up of the [1-13C]-pyruvate signal can be clearly observed, in the acquisition of a series of small flip-angle 13C MR spectra. The [1-13C]-pyruvate signal can also be detected using the cells lysate. An advantage of using lactate as hyperpolarized tracer for in vivo studies of metabolic processes is that it can be injected at nearly physiological concentration [5], that is relatively high (in the mM range), compared with that of pyruvate. In this work, we show that it is possible to go straight to 13C hyperpolarized lactate by the PHIP-SAH route and that hyperpolarized metabolites, obtained by means of parahydrogen method, can be applied to metabolic studies.Acknowledgements
The Italian Association for Cancer Research (AIRC, 2015 TRIDEO call) and Regione Piemonte (PAR FSC 2007/13) are gratefully acknowledged for financial support. We acknowledge the support and advices from Aspect Imaging scientists (Yoram Cohen and Peter Bendel).References
(1) Golman K., Axelsson O., Johannesson H., Mansson S., Olofsson C., Petersson J. S., Parahydrogen-induces polarization in Imaging: subsecond 13C angiography. Magn. Reson. Med. 2001, 46, 1-5.
(2) Ardenkjaer-Larsen H., Fridlund B., Gram A., Hansson G., Hansson G. L., Lerche M.H., Servin R., Thaning M., Golman K., Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc. Natl. Acad. Sci. 2003, 100, 10158-10163.
(3) F. Reineri, T. Boi, S. Aime, ParaHydrogen Induced Polarization of 13C carboxylate resonance in acetate and pyruvate. Nat. Commun. 2015, 6, 5858.
(4) F. Reineri, A. Viale, S. Ellena, T. Boi, V. Daniele, R. Gobetto, S. Aime, Use of Labile Precursors for the Generation of Hyperpolarized Molecules from Hydrogenation with Parahydrogen and Aqueous-Phase Extraction. Angew. Chem. Int. Ed. 2011, 50, 7350 –7353.
(5) Kennedy B. W. C., Kettunen M. I., Hu D.-E., Brindle K. M., Probing Lactate Dehydrogenase Activity in Tumors by Measuring Hydrogen/Deuterium Exchange in Hyperpolarized l-[1-13C,U-2H]Lactate. J. Am. Chem. Soc. 2012, 134, 4969-4977.
Figures