3704
Simple, cost-efficient, and highly sensitive molecular imaging with hyperpolarized milliTesla MRI1Department of Chemistry, Duke University, Durham, NC, United States, 2Chemistry, Duke University, Durham, NC, United States, 3School of Physics, University of Sydney, Sydney, Australia, 4A. A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 5Department of Physics, Harvard University, Cambridge, MA, United States, 6Departments of Physics, Radiology and BME, Duke University, Durham, NC, 7Harvard Medical School, Boston, MA, United States
Synopsis
Hyperpolarized MRI is a powerful approach to
PURPOSE
Hyperpolarized imaging with dissolution DNP and superconducting MRI scanners is cumbersome, slow and expensive. We describe a milliTesla (mT) approach to high-sensitivity hyperpolarized MRI that is simple, fast, and available at modest cost. In addition, we exploit longer relaxation times attainable for hyperpolarized compounds at low fields, enabling biomolecular imaging over much longer timescales than currently possible.
METHODS
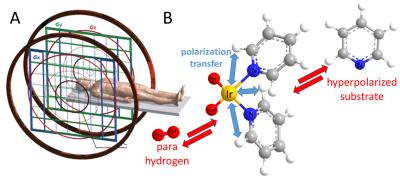
We combine low-cost, low field electromagnet-based MRI with parahydrogen based hyperpolarization. Specifically, we use a 6.5 mT electromagnet1 as depicted in Fig.1A and combine it with SABRE (Signal Amplification By Reversible Exchange), a simple hyperpolarization technique that produces hyperpolarized substrates directly in room temperature liquids. SABRE uses parahydrogen as hyperpolarization source, which is readily produced. The hyperpolarization is transferred from parahydrogen to other molecules, metabolites and drugs by the SABRE process.2 As seen in Fig. 1B, SABRE uses an Iridium based catalyst, which binds, both, parahydrogen and substrate to enable hyperpolarization transfer. The hyperpolarized markers can be imaged directly in solution,3 or injected into animals or patients for non-invasive molecular imaging.4 Hyperpolarization of protons (1H) in SABRE substrates is optimized at 6.5 mT because of nuclear spin energy level mixing of parahydrogen derived hydrides and substrate protons at exactly this field. Therefore, direct proton detection at this specific mT field is ideal. Nonetheless, protons are associated with relatively short hyperpolarization lifetimes, of order of seconds. Therefore, we also developed a SABRE variant to hyperpolarize heteronuclei (13C, 15N) which is associated with much longer lifetimes (many minutes). This alternative SABRE modality, known as SABRE-SHEATH (SABRE in SHield Enables Alignment Transfer to Heteronuclei), requires even lower µT-fields to enable energy level mixing and hyperpolarization transfer.5 With SABRE-SHEATH we create nitrogen-15 hyperpolarization with particularly long decay time constants, of above 20 min. at mT fields, enabling hour long molecular tracking of low concentration analytes.6RESULTS
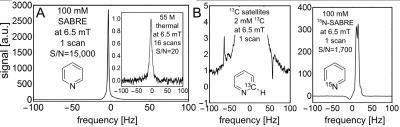
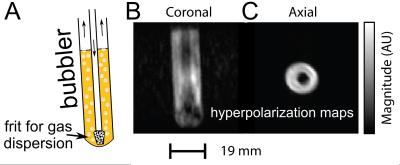
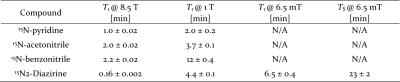
First, we compared hyperpolarized signals from 100 mM pyridine (with 5 protons per molecule) to signals from thermally polarized 55 M water (with 2 protons per molecule) both acquired at 6.5 mT. The results depicted in Fig. 2A show a 3,000 fold larger signal for the hyperpolarized solution. This corresponds to a proton polarization of 1.4%, and a signal enhancement of 1,650,000 when taking the concentrations into account. In order to demonstrate that it is possible to retain chemical specificity even at the low magnetic fields, where chemical shifts are not easily detectable, we looked at 13C containing pyridine at natural abundance (1.1% for 13C) as well as at 15N enriched pyridine. The resulting structured signals are displayed in Fig 2B. The 1H-13C and 1H-15N J-couplings cause characteristic line splitting in the low-field spectra allowing for chemical identification despite the lack of chemical shift resolution. Next, we performed imaging of a hyperpolarized solution as depicted in Fig. 3A. The image was performed by a standard FLASH sequence on a 100 mM solution of pyridine while 50% parahydrogen is bubbled through the phantom at atmospheric pressure. In Fig. 3B it is easily possible to identify the imaged object with a resolution of 1 x 1 mm2 displaying the gas dispersion tube (bubbler) with motion artifacts caused by the bubbling. Fig. 3C shows a projection image of the imaged bubbler. Finally, we performed nitrogen-15 hyperpolarization by SABRE-SHEATH on a range of molecules and examine hyperpolarization lifetimes at various magnetic fields; specifically, we compare experiments at high fields of 8.5 T to lower fields of 1T and 6.5 mT. As shown in the table depicted in Fig. 4, we find significantly extended signal lifetimes at the lower fields.DISCUSSION
The results indicate two important points; first, for hyperpolarized magnetic resonance (spectroscopy or imaging), signal-to-noise does not strongly depend on magnetic field. This counterintuitive result bucks trends in NMR or MRI, which are moving towards ever stronger and more expensive magnets. Second, the data strongly suggests that moving to lower, cheaper magnetic fields is associated with significant gains in hyperpolarization lifetime. Therefore, we expect low field magnetic resonance with hyperpolarization to outperform traditional platforms, not only in price, but also in performance when probing slower processes.CONCLUSION
In future research we expect first demonstrations of in vivo molecular imaging using the SABRE – low field combination. We also expect that other researchers may become interested in this approach because the barrier to entry is low, given the relative simplicity and affordability of the required hardware. The described technology may significantly alter point-of-care clinical practice because it offers molecular imaging for the price of an X-Ray.Acknowledgements
We acknowledge the support from Duke Arts and Sciences.References
1. Sarracanie M, LaPierre C D, Salameh N, Waddington D E, Witzel T, Rosen M S, Low-Cost High-Performance MRI. Sci. Rep. (2015); 5, 15177.
2. Adams R W, Aguilar J A, Atkinson K D, et al. Reversible interactions with para-hydrogen enhance NMR sensitivity by polarization transfer. Science (2009); 323, 1708-1711.
3. Hövener J B, Schwaderlapp N., Lickert T, et al. A hyperpolarized equilibrium for magnetic resonance. Nat. Commun. (2013); 4 2946.
4. Nelson S J, Kurhanewicz J, Vigneron D B, et al. Metabolic Imaging of Patients with Prostate Cancer Using Hyperpolarized 1-C-13 Pyruvate. Sci. Transl. Med. (2013), 5, 198ra108.
5. Theis T, Truong M L, Coffey A M et al, Microtesla SABRE Enables 10% Nitrogen-15 Nuclear Spin Polarization. J. Am. Chem. Soc. (2015); 137, 1404-1407.
6. Theis T, Ortiz G X , Logan A W J, et al. Direct and cost-efficient hyperpolarization of long-lived nuclear spin states on universal 15N2-diazirine molecular tags. Sci. Adv.(2016); 2, e1501438.
Figures