3703
Cost-efficient hyperpolarization of long-lived nuclear spin states on carbon-13 spin pairs1Department of Chemistry, Duke University, Durham, NC, United States, 2Departments of Physics, Radiology and BME, Duke University, Durham, NC, United States
Synopsis
Current hyperpolarization methods used in preclinical research are limited by high cost (>$2M) and short hyperpolarization lifetimes (<1 min). Here we demonstrate hyperpolarization of long-lived states with inexpensive equipment. Specifically, we use parahydrogen which is simple to produce (<$10k in equipment costs) and transfer its
INTRODUCTION
Hyperpolarized MRI is a powerful tool to study metabolic conversions in animals and patients.1 However, current technology is limited by high cost (>$2M) and short signal lifetimes (< 1 min). Therefore, we develop approaches that are cost-efficient and generate long-lasting hyperpolarization. This can be done by using parahydrogen, which can be produced with <$10k in equipment cost, and transfer its hyperpolarization to substrates that support long-lived singlet states.2 For this purpose, we recently used SABRE (Signal Amplification by Reversible Exchange)3 and hyperpolarized 15N2 spin pairs supporting hyperpolarization with lifetimes in excess of 20 min.4 Others recently used SABRE to hyperpolarize long-lived singlet states in 1H pairs5 and observed lifetimes extensions from 10 s to 50 s. Here we show that long-lived singlet states can also be hyperpolarized by SABRE in 13C2 pairs. This is significant because current preclinical and clinical hyperpolarized MRI exclusively uses hyperpolarized carbon-13.METHODS
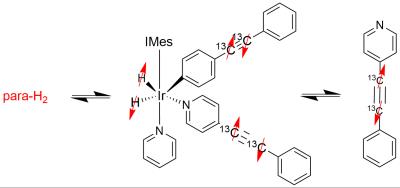
We produce parahydrogen by cooling normal hydrogen gas to about 37K in the presence of iron oxide and obtain ~90% enriched parahydrogen. This parahydrogen is bubbled through a solution at room temperature where polarization transfer is performed with a SABRE catalyst as depicted in Fig. 1. Parahydrogen and 13C labeled substrate reversibly bind to an iridium center. This establishes the hyperpolarization transfer complex. This complex has an average lifetime of about 50 ms. During this time, hyperpolarization is transferred from parahydrogen to substrate. Many repeated catalytic cycles build up hyperpolarization on the substrate (within ~ 5 min). Most critical to the hyperpolarization transfer is nuclear spin energy mixing of parahydrogen and substrate states. This mixing is established by going to very low magnetic fields where the frequency difference between 1H and 13C becomes comparable to the strength of J-coupling interactions in the system. Therefore, we perform the hyperpolarization process in a magnetically shielded environment established by mu-metal shields providing microTesla fields, about 200 times smaller than the Earth’s field. We introduced this approach as SABRE-SHEATH (SABRE in SHield Enables Alignment Transfer to Heteronuclei), initially focused on 15N hyperpolarization6, and expand it to carbon-13 in the present contribution. After hyperpolarization in the magnetic shield, the samples are transferred to an 8.5 T NMR magnet for detection. To measure decay time constants after hyperpolarization, the samples are kept at an intermediate field for variable time periods and subsequently transferred to the NMR spectrometer for detection.RESULTS
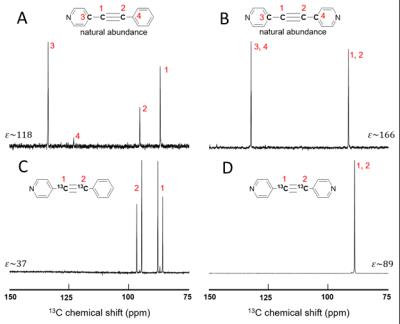
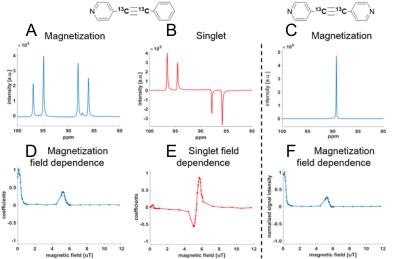
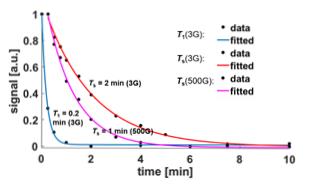
Figure 2 shows 13C SABRE-SHEATH for two compounds, symmetric bipyridyl acetylene and asymmetric pyridyl benzene acetylene. Both are pyridine derivatives that easily bind to the iridium center. We obtain between 40 to 200 fold enhancements over thermal measurements at 8.5 T. 13C-polarization is enhanced in samples with naturally abundant 13C (1.1%) or in 13C enriched samples. For the enriched samples, we specifically chose the acetylene group because those carbons display large mutual J-couplings (of ~180 Hz), ideal to support long-lived singlet states. On the examined compounds two types of hyperpolarization can be induced: magnetization and singlet order. This is illustrated in Fig. 3. We can easily distinguish between these two types of hyperpolarization. Magnetization is associated with in-phase signals as displayed in Fig. 3 A and C, whereas singlet order is associated with anti-phase signals as seen in part B. Also, we can select the hyperpolarization type by setting the magnetic field as indicated in Fig. 3 D-F. At very low fields of about 0.4 microT we obtain magnetization, whereas at slightly higher fields of ~6 microT we obtain primarily singlet order. Importantly, these two types of hyperpolarization are associated with different relaxation rates as displayed in Fig. 4. Magnetization decays with a T1 time of about 0.2 min (12 s) whereas singlet order can extend the hyperpolarization lifetime up to two minutes.DISCUSSION
Most importantly, the data shows that SABRE-SHEATH works for hyperpolarizing carbon-13. We can hyperpolarize either magnetization or singlet order by adjusting the magnetic field in the magnetically shielded environment. Singlet order can be highly advantageous because it displays slower relaxation such that the hyperpolarization can be observed on longer timescales; ideal for tracking and imaging of slower processes as is the case for many metabolic transformations.CONCLUSION
In future research hyperpolarization levels need to be increased and biologically relevant 13C labeled substrates need to be developed. Then, the cost-efficient SABRE-SHEATH modality will give researchers on modest budgets access to hyperpolarized probes for studying metabolism by non-invasive molecular imaging. Further down the road, we envision biomolecular imaging using SABRE-SHEATH at the cost of an X-Ray.Acknowledgements
We would like to thank the NSF (CHE-1363008) for financial support of this research.References
1. S. J. Nelson, J. Kurhanewicz, D. B. Vigneron, et al. Metabolic Imaging of Patients with Prostate Cancer Using Hyperpolarized 1-C-13 Pyruvate. Sci. Transl. Med. 5, 198ra108 (2013).
2. G. Stevanato, J. T. Hill-Cousins, P. Håkansson, et al. A Nuclear Singlet Lifetime of More than One Hour in Room-Temperature Solution. Angew. Chem. Int. Ed. 54, 3740-3743 (2015).
3. R. W. Adams, J. A. Aguilar, K. D. Atkinson, et al., Reversible interactions with para-hydrogen enhance NMR sensitivity by polarization transfer. Science 323, 1708-1711 (2009).
4. T. Theis, G. X. Ortiz , A. W. J. Logan, et al. Direct and cost-efficient hyperpolarization of long-lived nuclear spin states on universal 15N2-diazirine molecular tags. Sci. Adv. 2, e1501438 (2016).
5. S. S. Roy, P. J. Rayner, P. Norcott, et al. Long-lived states to sustain SABRE hyperpolarised magnetisation. Phys Chem Chem Phys 18, 24905-24911 (2016).
6. T. Theis, M. L. Truong, A. M. Coffey et al. Microtesla SABRE Enables 10% Nitrogen-15 Nuclear Spin Polarization. J. Am. Chem. Soc. 137, 1404-1407 (2015).
Figures