3701
A Referenceless Workflow for Hyperpolarized 13C EPI1Cancer Research UK Cambridge Institute, University of Cambridge, Cambridge, United Kingdom, 2Department of Biochemistry, University of Cambridge, Cambridge, United Kingdom
Synopsis
We have developed a workflow for hyperpolarized 13C EPI phase correction that requires no reference scan. The workflow provides ghost-free images on phantoms with large or tight fields of view and where there are multiple signal sources. Dynamic images acquired from hyperpolarized [1-13C]pyruvate and [1-13C]lactate in a tumor showed comparable image quality to those corrected using a separate 13C reference scan.
Purpose
Development of a referenceless workflow for hyperpolarized 13C EPI.Introduction
The development of DNP has enabled MRI studies of tissue metabolism, using hyperpolarized 13C-labelled substrates, at relatively high spatial and temporal resolutions 1. The transient nature of the hyperpolarization requires fast imaging. While EPI is an appealing technique because it can acquire a 2D image after a single excitation, it suffers from Nyquist ghosting. To correct for this a 13C reference scan can be acquired, but this takes up one frame of the dynamic data set. Alternatively a reference scan can be acquired using the 1H signal 2, but this can be suboptimal for 13C phase correction. Here we describe a workflow for hyperpolarized 13C EPI that renders reference scans unnecessary.Methods
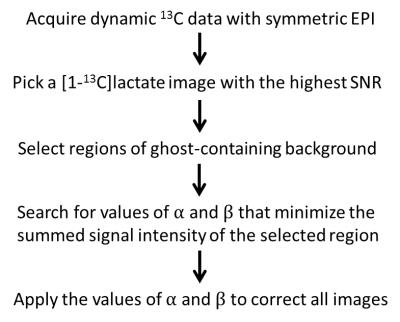
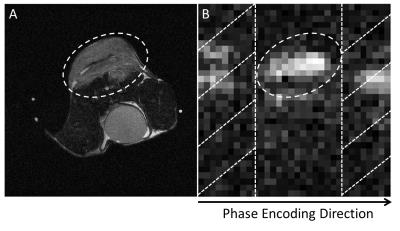
The workflow is illustrated in Figure 1, and is demonstrated using imaging of hyperpolarized [1-13C]pyruvate and [1-13C]lactate in vivo. An exhaustive search of the phase error correction coefficients is performed on the 13C EPI image to minimize signal in the ghost-containing background, which is readily obtainable from the proton image of tissue anatomy. As shown in Figure 2, a tumor ROI is selected from the proton image and mapped onto the [1-13C]lactate echo planar image, while regions outside the ROI in the phase encoding direction are treated as ghosting-containing background. The search is performed on the [1-13C]lactate image with the highest SNR and the correction coefficients obtained applied to all other images and for both metabolites.Experiments
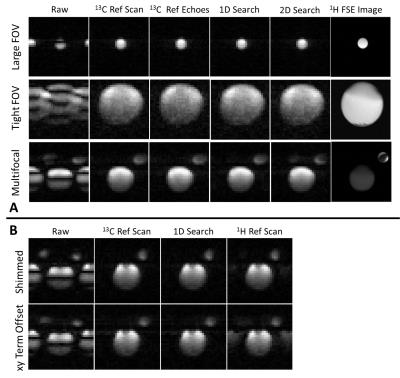
Experiments were performed on a 7T animal scanner (Agilent). Gradient-echo echo planar images were acquired from phantoms (diameters 7 mm and 18 mm, filled with 8.5M and 4M [1-13C]lactate respectively at thermal equilibrium) with a 4 cm FOV in order to examine performance of the method under conditions where there was a regular FOV, a tight FOV, and in the situation where there were multifocal signals. Image results from the workflow with 1D and 2D searches for the phase correction coefficients were compared with those corrected using a separate 13C reference scan and using 13C reference echoes 3. The workflow was also compared with the 1H reference scan method in the case of multifocal-signals, with a well-shimmed magnetic field and with a background gradient of 6mT/m2 in the XY direction. Imaging in vivo was conducted using the same parameters on female C57BL/6J EL4 lymphoma tumor-bearing mice following injection of hyperpolarized [1-13C]pyruvate, with alternating SpSp pulses on the [1-13C]pyruvate and [1-13C]lactate resonances and with a time resolution of 2s per metabolite.Results
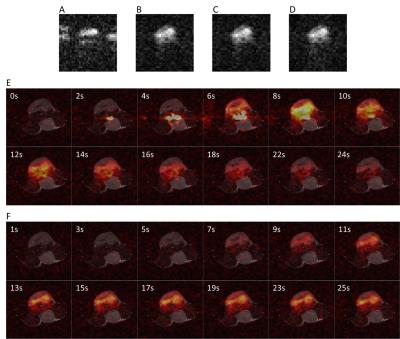
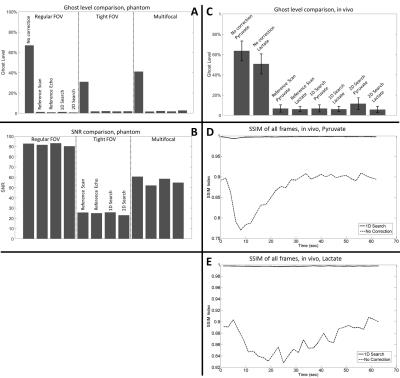
The proposed workflow resulted in phantom images of similar quality to those obtained using a separate 13C reference scan and using integrated 13C reference echoes (Figures 3A). The workflow also provided similar ghost-free images in vivo compared to those corrected with 13C reference scan (Figure 4). Quantitative measurements of residual ghosting in both phantom and in vivo images are shown in Figure 5A&5C. The workflow also showed an SNR benefit over the integrated echo method because of the prolonged TE with the latter method. The SNR was 1.7%, 3.0%, and 12.6% higher than the integrated echo method for a regular FOV, tight FOV, and with multifocal-signals respectively (Figure 5B). As shown in Figure 3B, the workflow gave better results than the 1H reference scan method (residual ghosting 1.3% versus 4.4%) under well-shimmed condition and this difference was increased in a de-shimmed condition (residual ghosting 1.6% versus 13.7%). The in vivo images obtained using the 1D search method and images corrected using 13C reference data were almost identical (SSIM≈1) through the whole dynamic imaging window for both [1-13C]pyruvate and [1-13C]lactate images (Figures 5D&5E).Discussion and Conclusion
The referenceless workflow resulted in ghost-free hyperpolarized 13C echo planar images of phantoms and in vivo. The resultant image quality was comparable to that obtained using a 13C reference scan or integrated 13C reference echoes and was consistently better than that obtained using 1H reference data, especially under suboptimal conditions. It also showed an SNR benefit over the integrated 13C reference echo method, and this benefit is expected to be enhanced in vivo because of the shorter T2*. The 1D search showed higher efficiency than the 2D search with similar ghost-removal capability and much reduced processing time. The workflow is compatible with 3D EPI and parallel imaging, and is readily translatable to clinical applications given the sparse nature of hyperpolarized 13C metabolite data, which will nearly always provide a ghost-containing background.Acknowledgements
No acknowledgement found.References
[1] Brindle KM. Imaging metabolism with hyperpolarized 13C labeled cell substrates. J Am Chem Soc 2015; 137:6418-6427
[2] Gordon, JW, Vigneron DB, Larson PEZ. Development of a symmetric echo planar imaging framework for clinical translation of rapid dynamic hyperpolarized 13C imaging. Magn Reson Med 2016; doi: 10.1002/mrm.26123
[3] Miller JJ, Lau AZ, Teh I, Schneider JE, Kinchesh P, Smart S, Ball V, Sibson NR, Tyler DJ. Robust and high resolution hyperpolarized metabolic imaging of the rat heart at 7 T with 3D spectral-spatial EPI. Magn Reson Med 2016; 75: 1515-1524
Figures