3700
Repeatability of quantitative hyperpolarized 13C MRSI measures of renal metabolism: impact of flow-sensitive gradients1Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 2Morgridge Institute for Research, Madison, WI, United States, 3Laboratory for Optical and Computational Instrumentation, University of Wisconsin-Madison, Madison, WI, United States, 4Radiology, University of Wisconsin-Madison, Madison, WI, United States, 5Biomedical Engineering, University of Wisconsin-Madison, Madison, WI, United States
Synopsis
Real-time quantification of in vivo metabolism with hyperpolarized 13C magnetic resonance spectroscopic imaging (MRSI) is currently limited by partial volume effects from intense vascular signal. Flow-sensitive, bipolar gradients are an attractive option for suppressing vascular signal due to their minimal influence on static spins. This work looks at the impact of incorporating bipolar gradients on the quantification and repeatability of hyperpolarized 13C MRSI metabolic measures of lactate-to-pyruvate area-under-the-curve ratios (AUCratio). The results suggest that incorporating bipolar gradients mitigates vascular partial voluming, increasing measured AUCratio, while reducing measurement repeatability, indicated by the larger repeatability coefficients.
Purpose
Real-time assessment of in vivo metabolism has been made possible by dynamic nuclear polarization (DNP)1, enabling magnetic resonance spectroscopic imaging (MRSI) of hyperpolarized 13C-labeled substrates. Despite the significant signal enhancement offered by DNP, MRSI still suffers from the low biological concentration and low gyromagnetic ratio, γ13C, of 13C nuclei.1 Therefore, to generate sufficient SNR, hyperpolarized 13C MRSI is generally acquired with a poorer spatial resolution than its anatomical 1H counterpart. This can cause significant partial voluming that may impact the accuracy and repeatability of metabolic measures. This is of particular concern for structures near major vessels containing high concentrations of intravenously injected hyperpolarized 13C substrate. Flow-sensitive bipolar gradients have been used to suppress hyperpolarized 13C vascular signal, thereby reducing partial volume effects.2,3 This work investigates the impact of bipolar gradients on the quantification and repeatability of hyperpolarized 13C MRSI metabolic measures.Methods
To evaluate test-retest repeatability of hyperpolarized 13C MRSI of renal metabolism, four, non-fasted ICR mice were assessed four times each: twice with bipolar gradients and twice without. Imaging sessions for each mouse were performed 2-4 days apart, with each mouse receiving a unique permutation of bipolar gradient state (on/off) across all sessions. All experiments complied with institutional animal care and use committee regulations.
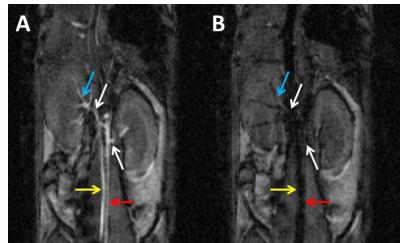
Imaging was performed on a 4.7T small animal scanner (Agilent, Palo Alto, CA) using a dual-tuned 1H/13C volume coil (Doty Scientific, Columbia, SC). T2-weighted, 1H FSE images (resolution=0.25×0.25mm2, THK=2mm, TR/TEeff=3500/66ms) were acquired for anatomical reference. T1-weighted, 1H SPGR images (resolution=0.25×0.25mm2, THK=1mm, TR/TE=45.9/11.7ms, FA=50°) were acquired for 12 bipolar gradient settings (Gmax=90mT·m-1, lobe duration=0-3.21ms, lobe separation=0ms, m1=0-939mT·ms2·m-1) to empirically determine which settings produced the most vascular signal suppression with the least renal signal loss for each mouse (Figure 1). For 13C imaging, bipolar gradient amplitudes were scaled by γ1H/γ13C to account for increased phase accrual of 1H vs. 13C nuclei.
For metabolic imaging, 30μL aliquots of [1-13C]pyruvic acid (Cambridge Isotope Laboratories Inc., Tewksbury, MA) doped with 15mM trityl radical (Oxford Instruments, Concord, MA) were polarized (12-20%) via DNP (HyperSense, Oxford Instruments, UK) and 10μL/g, less 100μL dead volume, was injected into the tail vein. Dynamic, hyperpolarized 13C images were acquired using a single-shot, multi-echo spiral sequence (resolution=2×2mm2, THK=3mm, TR/TE/ΔTE=110/7.5-8.4/1.19ms, echoes=5, FA=10º) either with or without bipolar gradients (Gmax=360mT·m-1, lobe duration=2.95-3.13ms, m1=3286-3689mT·ms2·m-1), and interleaved with slice-selective spectra (FA=5º) at ~5s temporal resolution. To maximize acquisition efficiency, no respiratory gating was used. Spiral data was corrected for RF excitation and readout trajectory deviations4. An iterative, least-squares estimation technique5 was used to reconstruct individual metabolite images. Parametric maps of lactate-to-pyruvate area-under-the-curve ratios (AUCratio)6 were generated after removing frames exhibiting low lactate SNR with pyruvate cross-talk. Two-way repeated measures ANOVA was used to test for differences in metabolic quantification due to bipolar gradient application. Measurement repeatability was assessed using the repeatability coefficient.7
Results
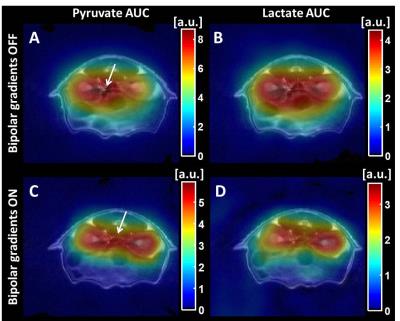
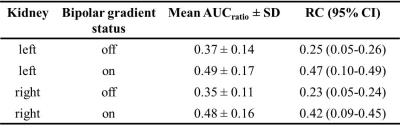
1H coronal images demonstrated successful vascular signal suppression in the abdominal aorta, vena cava, and renal and segmental arteries for the empirically selected bipolar gradient settings (Figure 1). Successful suppression of hyperpolarized 13C-pyruvate signal in the abdominal aorta was also achieved, increasing spatial co-registration of renal anatomy and metabolites (Figure 2). Application of bipolar gradients resulted in a significantly changed AUCratio in the right kidney (p=0.0282, α=0.05), with a similar trend of increasing AUCratio in the left kidney (p=0.1368). However, repeatability coefficients were larger in both kidneys when bipolar gradients were applied (Table 1).Discussion
Incorporating bipolar gradients to dephase 13C vascular spins and reduce partial voluming elicited larger AUCratio measures, consistent with previous findings.2 The lack of a statistically significant difference in the left renal AUCratio may be due to right/left differences in renal perfusion8 modulating the achievable vascular suppression by bipolar gradients. Respiratory motion may also impact right and left renal motion differently, causing variable diffusion-weighting and renal signal loss due to bipolar gradients. This could result in different AUCratio measurement variability in right and left kidneys.
Incorporation of bipolar gradients also resulted in higher AUCratio repeatability coefficients. This may be attributable to improved sensitivity to metabolic shifts in perfusion and metabolism arising from different blood-glucose concentrations9, or increased sensitivity to motion due to diffusion-weighting. Additional studies are planned to investigate the impact of respiratory gating and fasting on repeatability of measurements with and without bipolar gradients applied.
Conclusion
This study demonstrated that bipolar gradients can suppress hyperpolarized 13C vascular signal that may obfuscate metabolism in the anatomy of interest, resulting in improved measures of the AUCratio, but at the cost of reduced repeatability.Acknowledgements
This project received support from an AAPM Graduate Fellowship award, the Department of Medical Physics at the author's institution, and GE Healthcare.References
1. Ardenkjaer-Larsen J, Fridlund B, Gram A, et al. Increase in signal-to-noise ratio of >10,000 times in liquid-state NMR. Proc Natl Acad Sci. 2003;100(18):10158-10163.
2. Gordon J, Niles D, Adamson E, et al. Application of flow sensitive gradients for improved measures of metabolism using hyperpolarized 13C MRI. Magn Reson Med. 2016;75(3):1242-1248.
3. Lau A, Miller J, Robson M, et al. Cardiac perfusion imaging using hyperpolarized 13C urea using flow sensitizing gradients. Magn Reson Med. 2016;75(4):1474-1483.
4. Duyn J, Yang Y, Frank J, et al. Simple correction method for k-space trajectory deviations in MRI. J Magn Reson. 1998;132(1):150-153.
5. Reeder S, Brittain J, Grist T, et al. Least-squares chemical shift separation for 13C metabolic imaging. J Magn Reson Im. 2007;26(4):1145-1152.
6. Hill D, Orton M, Mariotti E, et al. Model free approach to kinetic analysis of real-time hyperpolarized 13C magnetic resonance spectroscopy data. PLoS One. 2013;8(9):e71996.
7. Bartlett J, and Frost C. Reliability, repeatability and reproducibility: analysis of measurement errors in continuous variables. Ultrasound Obstet Gynecol. 2008;31(4):466-475.
8. Kurklinsky A and Rooke T. Nutcracker phenomenon and nutcracker syndrome. Mayo Clin Proc. 2010;85(6):552-559.
9. Serrao E, Rodrigues T, Gallagher F, et al. Effects of fasting on serial measurements of hyperpolarized [1-13C]pyruvate metabolism in tumors. NMR Biomed. 2016;29(8):1048-1055.
Figures