3694
Dynamic Nuclear Polarization across the barrier: a Focused Ultrasound approach1Radiology and Nuclear Medicine, Radboud university medical center, Nijmegen, Netherlands
Synopsis
The delivery of targeted metabolic compounds can be hindered by a natural barrier in the brain. Nowadays, focused ultrasound techniques allow temporarily opening of this blood-brain barrier (BBB). Here we investigated the feasibility to combine this technique with dynamic imaging of hyperpolarized (HP) pyruvate. After opening the BBB in a focal spot, we acquired in vivo HP pyruvate images of the mouse brain using a dynamic gradient-echo imaging acquisition scheme at 7T. This approach eventually allows investigations with hyperpolarized compounds in the brain that are usually hindered by the BBB or suffer from a too slow uptake for DNP-imaging.
Introduction
The blood-brain barrier (BBB) is a protective layer of cells connected by tight junctions around blood vessels in the brain. This natural barrier prevents drugs from entering the brain. The same could hold for delivery of hyperpolarized (HP) 13C labeled agents used to assess dynamic metabolic information in the brain. Park et al. previously reported a lower uptake of HP pyruvate in the healthy rat brain, possibly due to hindrance from the BBB1. This problem might become even more severe for larger molecules. In this proof of concept we demonstrate the feasibility of combining opening of the BBB by focused ultrasound (FUS)2 with HP 13C MRI using dynamic nuclear polarization (DNP). In the near future, this approach might reveal the influence of the BBB on the uptake of HP agents or alterations in metabolism due to FUS-mediated BBB opening.Materials and Methods
All experimental protocols were approved by the National Animal Research Authority. The HP 13C-imaging protocol was evaluated in five mice and combined with FUS-mediated BBB opening in two other mice. All experiments were conducted on a 7T animal system (Clinscan, Bruker).
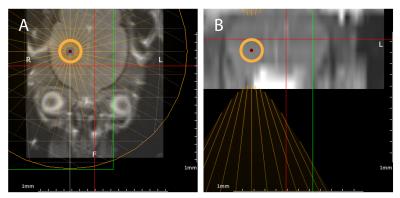
For BBB opening, animals were anesthetized and positioned in a dedicated MR-guided FUS set-up (IGT, France). MR reference images were acquired for ultrasound targeting in the left frontal lobe of the brain (fig1). The sonication (duration=120s, 10ms bursts, burst frequency=1Hz) was performed immediately after the injection of ultrasound microbubbles inside the MR system (Sonovue, Bracco, 500 µl/kg) using a 650 kHz focused transducer (focal spot 3.5 x 22 mm).
Subsequently the FUS set-up was replaced witha set-up for 13C-imaging, containing a dedicated 13C surface coil inside a small 1H volume coil for anatomical reference imaging. This set-up and the following methods were also used for 13C imaging evaluation in five mice.
[1-13C]-pyruvate was polarized in an in-house-built polarizer as described previously3. The polarized substrate was rapidly dissolved in a 1xPBS buffer solution to a final concentration of 80mM. 300µl HP pyruvate was injected within 15s after dissolution.
For dynamic imaging we used a slice selective gradient echo sequence (fig.2) to enable simultaneous pyruvate and lactate imaging. To this end, the MR-system carrier frequency was set in between the pyruvate and lactate resonance frequencies. The use of a readout bandwidth of 60Hz/px resulted in complete separation of both signals by their chemical shift dispersion as described by Steinseifer et al4. 13C-GRE magnitude images were acquired every 1.6s (FOV=50mm, 32x16 in-plane resolution, TR=100ms, TE=9.4ms, flip angle=10°). A phantom containing [1-13C]-pyruvate and [1-13C]-lactate doped with TEMPOL radical was placed next to the animal to obtain a 13C reference image (similar settings, except: NA=32, TR=500ms, flip angle=90°) for image registration. The concentration curves of pyruvate and lactate were visualized by plotting the total signal intensity over time of a region of interest. Data were not corrected for T1-decay and signal loss due to RF pulsing.
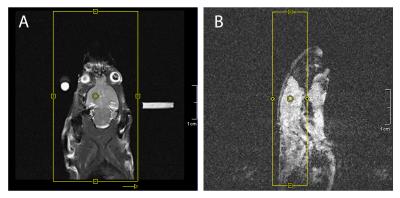
Since Gd-based contrast agents cannot cross the intact BBB, Magnevist (0.25 ml/kg) was injected after 13C-imaging to verify opening of the BBB using pre- and post-contrast T1-weighted MR imaging.
Results
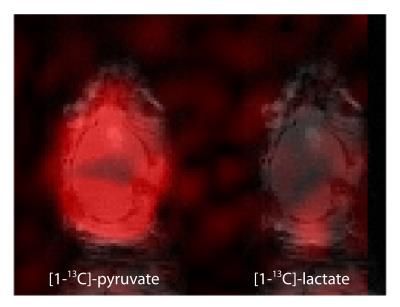
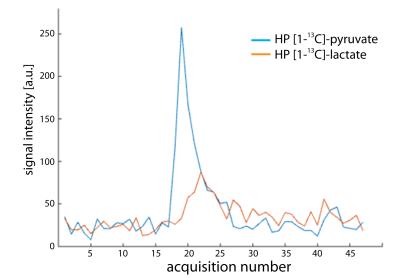
Fig.3 shows an example of an image of HP pyruvate and lactate that was simultaneously acquired in vivo in the mouse brain without BBB opening. Metabolite signals were separated over a distance of half the FOV and were mapped to the reference image. A representative dynamic time evaluation of the HP signals is shown in fig.4.
Pre- and post-contrast images in fig.5 confirm a successful opening of the BBB, as we observed a hyperintense region where the brain was sonicated. However, the pyruvate image did not show any evident signal intensities that can be related to BBB-opening.
Discussion and Conclusion
In this study we successfully showed the feasibility of combining BBB opening using FUS with dynamic imaging of hyperpolarized 13C-labeled compounds. The BBB was opened prior to, and remained open during our HP 13C-imaging experiment, as confirmed by contrast-enhanced MRI. However, SNR of the HP signal is currently too low to draw conclusions upon enhanced uptake or release of HP metabolites in the targeted brain region. There is still room for improving polarization levels and further optimization of the imaging parameters. Gaining SNR by enlarging the voxel size and/or FOV is only possible at the cost of losing in-plane resolution.
Despite challenges that have to be overcome, this approach could enable the delivery of HP agents that suffer from the absence of suitable transporters in the brain or from low uptake across the BBB, creating possibilities to explore metabolic pathways in the brain that were not within reach up to now.
Acknowledgements
No acknowledgement found.References
1. Park, I. et al. Hyperpolarized 13C magnetic resonance metabolic imaging: application to brain tumors. Neuro. Oncol. 12, 133–44 (2010).
2. Hynynen, K., et al. Noninvasive MR Imaging–guided Focal Opening of the Blood-Brain Barrier in Rabbits. Radiology 220, 640–646 (2001).
3. Breukels, V. et al. Direct dynamic measurement of intracellular and extracellular lactate in small-volume cell suspensions with (13)C hyperpolarised NMR. NMR Biomed. 28, 1040–8 (2015).
4. Steinseifer, I. et al. Metabolic imaging of multiple X-nucleus resonances. Magn. Reson. Med. 70, 169–175 (2013).
Figures