3692
Multi-echo balanced steady state imaging of hyperpolarized [1-13C]-pyruvate at 9.4T1Department of Clinical Medicine, The MR Research Centre, Aarhus University Hospital, Aarhus N, Denmark
Synopsis
Hyperpolarized (HP) [1-13C]-pyruvate imaging has gained increasing attention, as its ability to monitor metabolic changes in real-time is expected to have a great clinical impact. In this work we investigate the use of a fast multi-echo balanced steady state free precession (BSSFP) sequence to acquire spectroscopic images at 9.4T. We show that this technique, in combination with the iterative Dixon type reconstruction technique (IDEAL), yields dynamic high resolution spectroscopic maps in a 1H phantom as well as for preliminary in vivo HP [1-13C]-pyruvate experiments in rat kidneys.
Purpose
Hyperpolarized (HP) [1-13C]-pyruvate imaging has gained increasing attention, as its ability to monitor metabolic changes in real-time is expected to have a great clinical impact. This is due to the inherent coupling of several disease states to the associated metabolic phenotype, and the treatment thereof1. Several imaging and spectroscopy methods have been proposed and adopted for various applications in both small and large animals, and more recently translated to human use2. In this work we investigate the use of a fast multi-echo balanced steady state free precession (BSSFP)3 sequence to acquire spectroscopic images using a 9.4T scanner. In combination with the iterative Dixon type reconstruction technique (IDEAL)4, it is expected that this approach will yield dynamic high resolution metabolic activity maps, following an injection of HP [1-13C]-pyruvate. We present the dynamic application of this technique at 9.4T in a 1H phantom as well as preliminary in vivo 13C results in rat kidneys.Materials and methods
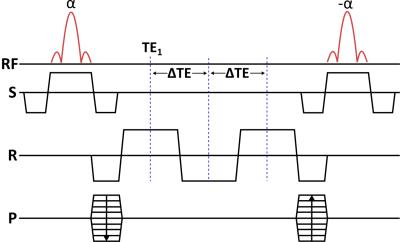
A multi-echo BSSFP sequence as illustrated in Figure 1 was implemented on a 9.4T pre-clinical MR system (Agilent, UK) equipped with a dual tuned 13C/1H volume rat coil (Doty scientific, US).
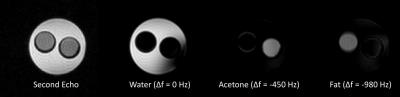
A 1H phantom containing water, acetone and vegetable oil was used to test the sequence and the subsequent IDEAL reconstruction, see Figure 2. Imaging parameters were: eight echoes with TE1 = 1.4 ms, ΔTE = 1.1 ms, TR = 10.1 ms, FOV = 5 x 5 cm2, slice thickness = 2 mm, BW = 100 kHz, acq. matrix = 32 x 32 and flip angle = 30°.
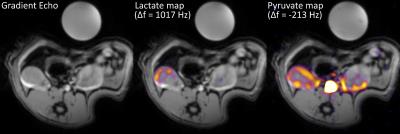
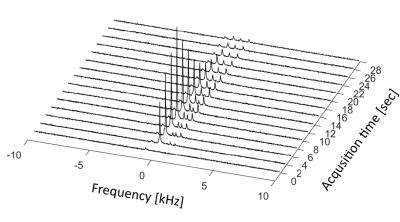
Subsequently, two HP [1-13C]-pyruvate experiments were performed using two healthy rats injected with 1 mL pyruvate mixture, irradiated with 139.93 GHz microwaves for >2.5 hours at 0.8° K, using a 5T GE SpinLab polarizer to a reproducible polarization above 40%5. A 13C HP imaging experiment was set up, encompassing the acquisition of a global 13C spectrum followed by the multi-echo BSSFP imaging sequence, repeated every two seconds after the injection of the HP metabolite. Parameters for the spectral acquisitions were: TR = 1.8 s, BW = 10 kHz and flip angle = 4°. Parameters for the multi-echo BSSFP acquisitions were: eight echoes with TE1 = 1.1 ms, ΔTE = 0.42 ms, TR = 6.1 ms, FOV = 7 x 7 cm2, slice thickness = 10 mm, BW = 192 kHz, acq. matrix = 32 x 32 and flip angle = 4°. Lastly, a set of axial multi-slice gradient echo images were acquired for overlaying the metabolic maps. Imaging parameters were: 16 slices with TR = 100 ms, TE = 2.6 ms and a small flip angle.
The subsequent first order phase correction and IDEAL reconstruction was performed in Matlab (MathWorks, Natick, MA, USA). The reconstructed metabolic maps were transferred to DICOM format and imported to OsiriX (Pixmeo SARL, Bernex, Switzerland) for image overlaying.
Results and discussion
Figure 2 shows the magnitude image of the second echo acquired using the multi-echo BSSFP sequence as well as IDEAL metabolite maps for water (on resonance), acetone (Δf = -450 Hz) and vegetable oil (Δf = -980 Hz) reconstructed at their respective frequencies. The generated metabolite maps show satisfactory suppression of the metabolites present, with some contributions due to imperfect shimming, confirming the feasibility of the approach. Figure 3 shows an axial gradient echo slice with both visible kidneys, as well as the same gradient echo image overlaid with preliminary in vivo reconstructed IDEAL metabolic maps deriving from the HP pyruvate (Δf = -213 Hz) and lactate (Δf = 1017 Hz) signals. The metabolic maps correspond to the acquisition performed eight seconds after the start of the HP pyruvate injection. The IDEAL lactate map show no residual signal in the aorta region, indicating the correct separation of the two metabolic species. The acquired global 13C spectrum shown in Figure 4 clearly shows the HP pyruvate (Δf = -213 Hz) injection and the subsequent exponential decay of the signal. Additionally, peaks corresponding to a bicarbonate phantom (Δf = -900 Hz), alanine (Δf = 360 Hz), pyruvate-hydrate (Δf = 620 Hz) and lactate (Δf = 1017 Hz) are also present.Conclusion
The preliminary data presented here demonstrate the use and feasibility of the seperation scheme employing multi-echo BSSFP and IDEAL reconstruction. Further more, the good seperation of the different 1H and 13C species combined with the high resolution and fast acquisition provided by multi-echo BSSFP imaging confirm the expectation that this scheme can be used to produce dynamic metabolite imaging for future in vivo HP experiments.Acknowledgements
No acknowledgement found.References
1 J. Kurhanewicz, D. B. Vigneron, K. Brindle, E. Y. Chekmenev, A. Comment, C. H. Cunningham, …, C. R. Malloy; Analysis of Cancer Metabolism by Imaging Hyperpolarized Nuclei: Prospects for Translation to Clinical Research. Neoplasia (New York, N.Y.), 13(2) (2011) 81–97.
2 C. H. Cunningham, J. Y. Lau, A. P. Chen, B. J. Geraghty, W. J. Perks, I. Roifman, G. A. Wright, K. A. Connelly; Hyperpolarized 13C Metabolic MRI of the Human Heart: Initial Experience. Circulation Research, (2016) CIRCRESAHA.116.309769.
3 J. Leupold, S. Månsson, J. S. Petersson, J. Hennig, O. Wieben; Fast multiecho balanced SSFP metabolite mapping of 1H and hyperpolarized 13C compounds. Magma, 22(4) (2009) 251-6.
4 F. Wiesinger, E. Weidl, M. I. Menzel, M. A. Janich, O. Khegai, S. J. Glaser, A. Haase, M. Schwaiger, R. F. Schulte; IDEAL spiral CSI for dynamic metabolic MR imaging of hyperpolarized [1-13C]pyruvate. Magn. Reson. Med., (2012), 68: 8–16.
5 P. M. Nielsen, E. S. Szocska Hansen, T. S. Nørlinger, R. Nørregaard, L. Bonde Bertelsen, H. Stødkilde Jørgensen, C. Laustsen; Renal ischemia and reperfusion assessment with three-dimensional hyperpolarized 13C,15N2-urea. Magn. Reson. Med., (2016), 76: 1524–1530.
Figures