3687
Simultaneous multislice acquisition without trajectory modification for hyperpolarized 13C experiments1Physical Sciences, Sunnybrook Research Institute, Toronto, ON, Canada, 2Medical Biophysics, University of Toronto, Toronto, ON, Canada, 3GE Healthcare, Toronto, ON, Canada
Synopsis
Recently we proposed using simultaneous multislice (SMS) acceleration to improve spatial coverage in the hyperpolarized 13C experiment, and demonstrated that controlled aliasing improves conditioning of the inverse problem. For single-shot experiments this requires gradient modulation along z, which can be sensitive to gradient imperfections. Here, we show that inherent coil sensitivity variations can be sufficient for SMS acceleration in hyperpolarized 13C experiments, without additional readout gradient modification. Two-fold acceleration with a 8 cm slice gap can be obtained with less than 20% SNR loss. We anticipate that this strategy will enable multiple organ 13C imaging of in vivo metabolism.
Purpose
To improve spatial coverage in hyperpolarized 13C experiments.Introduction
Hyperpolarized 13C MRI is a promising tool for non-invasive characterization of in vivo metabolism [1]. The feasibility of using this emerging technology in humans has recently been shown [2,3]. However, achieving extended volumetric coverage of multiple metabolites can be time consuming and may be difficult to achieve due to the relatively short in vivo lifetime of the 13C probe, and short (20 s) breath-hold durations. Recently we proposed using simultaneous multislice (SMS) acceleration [4-6] to improve spatial coverage and demonstrated that controlled aliasing improves conditioning of the inverse problem [7]. For single-shot experiments this requires gradient modulation along z, which can be sensitive to gradient imperfections. In this abstract, we investigate the possibility of using only coil sensitivity variations to reconstruct hyperpolarized 13C SMS data.Methods
Imaging acquisition
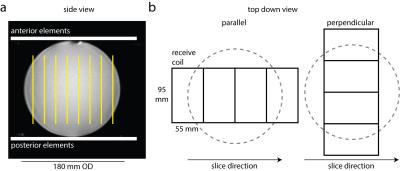
A GE MR750 3.0T MRI, with 13C volume transmit coil (GE Healthcare) and 13C 8-channel receive coil (2 rigid paddles, 20×24 cm2 each with four 5.5×9.5 cm2 elements oriented in a 1x4 array) was used for this study. The coil setup is shown in Figure 1. A spectrally-selective, single-shot spiral sequence (TR 5s, FOV 480 mm, readout duration 32 ms, in-plane resolution 7.5x7.5 mm2, 8 slices, slice thickness 20 mm, NEX 128) was used to image a 18 cm OD sphere containing ethylene glycol (99%, 0.36 M natural abundance 13C) [8]. The scan was repeated twice with the receive coil placed such that the linear row of elements within the paddle were oriented either parallel with or perpendicular to the imaging slices.
Coil sensitivity estimation.
Images were reconstructed by NUFFT. The ESPIRiT method was used to estimate coil sensitivities from low resolution images (20 mm isotropic) [9].
G-factor calculation.
G-factor maps for 2-fold slice acceleration were computed using $$$g_{i} = \sqrt{(S^{H}S)^{-1}_{i,i}(S^{H}S)_{i,i}}$$$, where i = 1,2 and S is an Ncoil x Nslice sensitivity matrix. The retained SNR (1/g) is reported. Pairwise g-factor maps were computed for each combination of summed slices.
Results
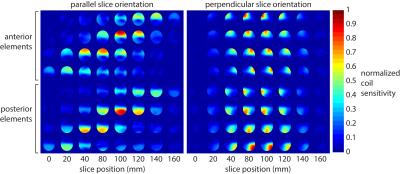
Figure 2 shows the coil sensitivity maps obtained using ESPIRiT. Placing the paddles with the elements oriented parallel to the prescribed imaging slices increases coil sensitivity variations between slices. Figure 3 shows retained SNR (1/g-factor) maps, which indicate improved conditioning of the parallel imaging reconstruction depending on coil orientation. Retained SNR maps are displayed in a triangular matrix. Diagonal sets indicate constant slice separation. A slice separation of 8 cm results in a retained SNR > 80%.Discussion
SMS impacts SNR via g-factor noise penalty only (i.e. SNRSMS = SNR/g) without a scan time reduction penalty. This 13C coil configuration (volume transmit with anterior/posterior receive array) has recently been used to image human cardiac metabolism [2]. These results show that the setup can be easily adapted to accelerate existing clinical 13C imaging methods. This approach is feasible as long as care is taken to orient the imaging slices parallel to the individual coil elements.
The strategy circumvents the need for additional gradient blips [6] in the through-slice direction, for which gradient timing errors can result in difficult-to-correct image artifacts. Inter-slice phase modulation may also be incompatible with thick slices (i.e. 1 cm slices relative to 7-8 cm slice gaps) which violate the controlled aliasing assumption of unique phases per simultaneously acquired slice.
Potentially, this will enable whole-body, large FOV imaging of multiple organs (i.e. heart, liver, brain) especially as extended high channel count 13C receiver arrays become available.
Conclusions
Inherent coil sensitivity variations were shown to be sufficient for SMS acceleration in hyperpolarized 13C experiments, without additional readout gradient modification. Two-fold acceleration with a 8 cm slice gap can be obtained with less than 20% SNR loss. We anticipate that this strategy will enable multiple organ 13C imaging of in vivo metabolism.Acknowledgements
No acknowledgement found.References
1. Ardenkjaer-Larsen JH et al. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. PNAS 2003 Sep 2;100(18):10158-63.
2. Nelson SJ et al. Metabolic imaging of patients with prostate cancer using hyperpolarized [1-¹³C]pyruvate. Sci Transl Med. 2013 Aug 14;5(198):198ra108.
3. Cunningham CH et al. Hyperpolarized 13C Metabolic MRI of the Human Heart: Initial Experience. Circ Res. 2016 Sep 15.
4. Larkman DJ et al. Use of multicoil arrays for separation of signal from multiple slices simultaneously excited. J Magn Reson Imaging. 2001 Feb;13(2):313-7.
5. Breuer FA et al. Controlled aliasing in parallel imaging results in higher acceleration (CAIPIRINHA) for multi-slice imaging. Magn Reson Med. 2005 Mar;53(3):684-91.
6. Setsompop K et al. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magn Reson Med. 2012 May;67(5):1210-24.
7. Lau AZ et al. Simultaneous multi-slice imaging of multiple metabolites using spectral-spatial excitation for hyperpolarized 13C experiments. Proc ISMRM 2014 (981).
8. Lau AZ et al. Rapid multislice imaging of hyperpolarized 13C pyruvate and bicarbonate in the heart. Magn Reson Med. 2010 Nov;64(5):1323-31.
9. Uecker M et al. ESPIRiT--an eigenvalue approach to autocalibrating parallel MRI: where SENSE meets GRAPPA. Magn Reson Med. 2014 Mar;71(3):990-1001.
Figures