3680
A comprehensive assessment of methods for combining phase data from array radio-frequency coils at 7 T1Department of Biomedical Imaging and Image-guided Therapy, Medical University of Vienna, Vienna, Austria, 2Donders Institute for Brain, Cognition and Behaviour, Radboud University Nijmegen, Netherlands, 3Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, MA, United States, 4Department of Neurology, University at Buffalo, NY, United States, 5MRI Clinical and Translational Research Center, University at Buffalo, NY, United States
Synopsis
Methods for combining phase data from array RF coils are quantitatively compared at 7 T. Of the reference-free approaches (which all leave arbitrary contributions to the total phase), the Virtual Reference Coil method yielded the best phase matching. The reference-free method COMPOSER removed non-B0-related phase but requires an artifact-free short echo-time reference measurement. Of the multi-echo methods, SVD, HIP and ASPIRE all had uniform phase matching. ASPIRE has higher CNR for a narrow range of echo times, but requires TE2=2 x TE1. The data and assessment scripts used in this study will be made publicly available.
PURPOSE
MRI methods based on the phase evolution in T2*-weighted sequences, such as Susceptibility-Weighted Imaging (SWI) and Quantitative Susceptibility Mapping (QSM), benefit from the increase in contrast-to-noise ratio (CNR) associated with high and ultra-high static magnetic field, B0, and the use of phased arrays of radiofrequency coils. The phase measured with each coil comprises a term which reflects the local deviation from B0 and evolves with time and other invariant contributions including the coil sensitivity. Recent years have seen a wealth of approaches to combining phase signals from array coils. These differ in computational complexity and acquisition requirements, with some requiring multi-echo data or a reference scan with a volume coil or the phased array itself. The quality of phase matching and CNR also vary widely. In this study we compare the quality of phase images calculated with a comprehensive range of methods. This extends prior work1 through the inclusion of additional methods developed very recently, the use of data better suited to phase difference-based methods and the use of sensitivity weighting in the phase combination.METHODS
High resolution 3D T2*-weighted
gradient-echo images of the brain of one male subject were acquired with a 7T MR whole body Siemens MAGNETOM scanner and a 32 channel head
coil (Nova Medical), with 0.5×0.5×0.9 mm3 voxels, TEs/TR ={[6.0,12.0,18.0,24.0,30.0,36.0]ms}/47
ms, TA=15'14. Low resolution single-echo GE data (2×2×3 mm3, TE=1.4 ms) were additionally acquired
with both the birdcage transceive coil (AC) and the receive array (VC) for the short
echo reference2 and the Roemer3 methods.ANALYSIS
High resolution phase data were combined over channels: $$\theta_{comb}=\angle\sum_jW_j\cdot M_j\cdot e^{-i(\theta_{cor,j})},$$ where $$$\angle$$$ is the angle operation (atan2), Wj the absolute sensitivity of coil j, Mj the magnitude and $$$\theta_{cor,j}$$$ the phase corrected with the relevant method. These were: i) "No Correction" ii) Scalar Phase Matching4 "SPM_reg" iii) improved Scalar Phase Matching "SPM_imp" iv) Virtual Reference Coil5 "VRC" v) reference transceive coil measurement as in Ref3 "Roemer" vi) dual-echo reference scan6 "MCPC-3D-II", with unwrapping with PRELUDE in 2D vii) short echo-time reference scan2 "COMPOSER" viii) Singular Value Decomposition7 "SVD" ix) Hermitian Inner Product8 "HIP" x) multi-echo calculation of offsets6 "MCPC-3D-I" and xi) ASPIRE, an approach presented in a separate submission to this annual meeting. ASPIRE is based on MCPC-3D-I, but avoids the need to unwrap phase images in the calculation of phase offsets by selecting TE2=2×TE1. Mj was taken as an estimate of the Wj for all methods other than SPM_reg, for which it was set to unity4, and Roemer, for which the sensitivity was estimated relative to the volume coil. The quality of phase matching achieved (the consistency of corrected values over coils) was assessed via the metric Q: $$Q=100\times\frac{\sqrt{abs\big(\sum_jW_j\cdot M_j\cdot e^{-i(\theta_{cor,j})}\big)}}{\sqrt{abs\big(\sum_jW_j\cdot M_j}\big)},$$ which ranges from 0 to 100 (ideal phase matching). The contrast-to-noise ratio (CNR) was also assessed in 12 neighboring gray and white matter regions.RESULTS
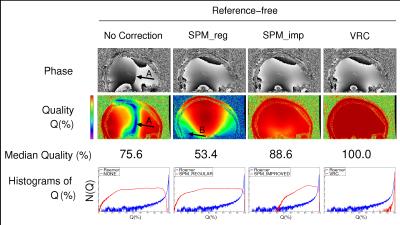
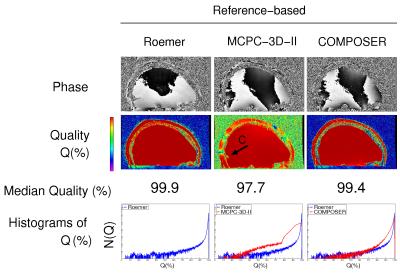
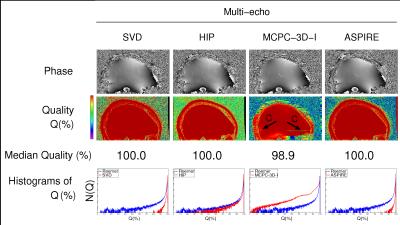
Phase images and Q maps are shown in Figure 1, 2 and 3 for the reference-free, reference-based and multi-echo approaches respectively. There were regions of complete signal destruction (Q=0) and an open-ended fringeline in the phase at the center of the image with No Correction (arrow A). Phase matching was poor in inferior regions with SPM_reg (B), and improved by the weighting introduced in SPM_imp. The reference-based methods all had some poorly matched voxels due to minor coregistration errors and shortcomings (such as Gibbs ringing) in the low resolution reference scans. MCPC-3D-I and MCPC-3D-II both had isolated inferior problems from phase unwrapping errors (C). SVD and HIP phase values were near identical (difference<0.1%). Phase matching was close to perfect in all voxels with VRC, Roemer, SVD, HIP and ASPIRE.DISCUSSION AND CONCLUSION
The choice of phase combination method depends on the experimenters acquisition requirements, but some methods with similar requirements outperformed others. In SPM_imp, nearby coils with similar phase profile were weighted, yielding improved phase matching compared to SPM_reg and providing a more robust virtual reference coil image for the VRC method. VRC had the highest Q value of the reference-free methods, all of which contained arbitrary non-B0-related phase. The SVD and HIP methods had high Q but reduced CNR if the range of echo times is narrow1 - an effect not found to be significant in this study. ASPIRE requires that TE2=2×TE1, but is simple, robust, and has no CNR penalty even for a narrow range of echo spacings. The VRC, Roemer, COMPOSER, SVD, HIP and ASPIRE methods all had high Q and CNR. This 7T ME-GRE data and assessment tools will be made publicly available to aid the objective assessment of other methods.Acknowledgements
This study was funded by the Austrian Science Fund (KLI 264). Additional support was provided by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR001412. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.References
1. Robinson SD, Bredies K, Khabipova D, et al. An illustrated comparison of processing methods for MR phase imaging and QSM: combining array coil signals and phase unwrapping. NMR Biomed 2016 doi: 10.1002/nbm.3601
2. Robinson SD, Dymerska B, Bogner W, et al. Combining phase images from array coils using a short echo time reference scan (COMPOSER). Magn Reson Med 2015 doi: 10.1002/mrm.26093
3. Roemer PB, Edelstein WA, Hayes CE, et al. The NMR phased array. Magn Reson Med 1990;16(2):192-225.
4. Hammond KE, Lupo JM, Xu D, et al. Development of a robust method for generating 7.0 T multichannel phase images of the brain with application to normal volunteers and patients with neurological diseases. NeuroImage 2008;39(4):1682-92.
5. Parker DL, Payne A, Todd N, et al. Phase reconstruction from multiple coil data using a virtual reference coil. Magn Reson Med 2014;72(2):563-9. doi: 10.1002/mrm.24932
6. Robinson S, Grabner G, Witoszynskyj S, et al. Combining phase images from multi-channel RF coils using 3D phase offset maps derived from a dual-echo scan. Magnetic Resonance in Medicine 2011;65:1638-48.
7. Khabipova D, Wiaux Y, Gruetter R, et al. A modulated closed form solution for quantitative susceptibility mapping--a thorough evaluation and comparison to iterative methods based on edge prior knowledge. NeuroImage 2015;107:163-74. doi: 10.1016/j.neuroimage.2014.11.038
8. Bernstein MA, Grgic M, Brosnan TJ, et al. Reconstructions of phase contrast, phased array multicoil data. Magn Reson Med 1994;32(3):330-4.
Figures