3662
Changes in brain iron concentration after exposure to high altitude hypoxia by quantitative susceptibility mapping1Department of Electronic Science, Xiamen University, Xiamen, People's Republic of China, 2Department of Communication Engineering, Xiamen University, Xiamen, People's Republic of China, 3Zhongshan Hospital, Xiamen, People's Republic of China, 4Department of Physiology and Neurobiology, Xiamen University, Xiamen, People's Republic of China
Synopsis
Environmental factors may influence brain iron concentration. We investigated the changes of magnetic susceptibility and R2* values of cerebral regions especially in six deep gray matter nuclei of twenty-nine participants after high altitude exposure for four weeks. The results show that the susceptibility values of gray matter, especially in caudate nucleus, putamen, globus pallidus, substantia nigra, red nucleus, increased significantly. Traditional R2* maps verify the results of QSM evaluation except in red nucleus. Therefore, high altitude hypoxia can lead to significant increase of cerebral iron concentration.
Purpose
While iron plays important roles in physiological functions and development of human brain, aberrant deposition of iron is often associated with toxic free radical and pathological damage.1 The pathologic mechanisms of iron deposition have been actively investigated, but possible environmental factors, which may influence brain iron concentration, has rarely been mentioned. Recent study discover that acute exposure to high altitude (HA) hypoxia can cause disruption of the blood-brain barrier (BBB) and thus iron deposition in cerebral regions.2 However, it has not been reported that whether prolonged acclimatization to HA can cause iron deposition in brain. In present work, we aimed to investigate the effects of HA acclimatization on iron concentration in cerebral tissue by quantitative susceptibility mapping (QSM) and traditional R2* measurement.Methods
Twenty-nine healthy participants (13 female, mean age = 20±0.8) were imaged on a 3T Siemens Tim Trio MRI scanner (Siemens, Erlangen, Germany) at sea level before their ascent to Qinghai-Tibet Plateau (4200 m) and immediately after they returned from an expedition for 4 weeks. All images were acquired with three-dimensional multi-echo gradient-recalled sequence. The experimental parameters were: TR = 55 ms, TE1 = 3.6 ms, 8 echoes at ΔTE = 5.9 ms, flip angle = 15°, slice thickness = 2 mm and acquisition matrix size = 256×256. The field map was estimated from the multi-echo datasets using a nonlinear fitting algorithm followed by spatial phase unwrapping and projection onto the dipole field was used for background field removal. Finally, QSM was obtained by using morphology enabled dipole inversion (MEDI) method.3 R2* maps were reconstructed by mono-exponential fitting with all echoes of the GRE magnitude images. To generate binary segmentation masks of cerebrospinal fluid (CSF), white matter (WM), gray matter (GM) (Fig. 1A), we utilized FMRIB’s automated segmentation tool (FAST) to process magnitude images of the first echo. We obtained the regions of interest (ROIs) including caudate nucleus (CN), putamen (PT), globus pallidus (GP), substantia nigra (SN), red nucleus (RN), and dentate nucleus (DN) by converting a customized common atlas created in the Montreal Neurological Institute (MNI) space to each individual participant space using FMRIB Software Library (FSL, University of Oxford) (Fig. 1B). Paired t-tests were performed to examine the differences of iron concentration in cerebral regions.Result
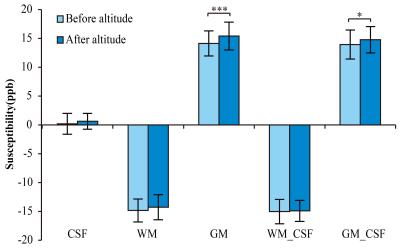
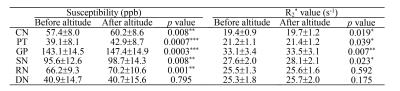
This work shows that no significant variation can be observed in the susceptibility value of CSF after HA exposure. Similarly, the susceptibility value of WM shows no significant difference after altitude, no matter with or without referencing the susceptibility value of CSF to zero. However, the susceptibility value of GM increases significantly after HA exposure with (p < 0.05) and without (p < 0.001) referencing the susceptibility value of CSF to zero (Fig. 2A). All susceptibility values in the six GM nuclei relative to CSF (0.00 ppb) were examined. The susceptibility values in CN, SN, RN (p < 0.01), PT and GP (p < 0.001) regions of 29 participants increase significantly after HA exposure in comparison with counterpart in before-altitude group. Changes of the R2* values within CN, PT, GP and SN regions verify the results of QSM evaluation. However, the R2* value within the RN region fails to demonstrate statistical difference between before- and after-altitude groups (Table 1).Discussion
Tissue iron serves as the dominant source of magnetic susceptibility in GM.4 The recovery of oxygen saturation (SaO2) after HA exposure in our clinical examination eliminates the interference from SaO2 in brain iron investigation. Therefore, the results indicate that iron levels increase in GM, especially in CN, PT, GP, SN and RN nuclei with potential causes of increased degradation of hemoglobin, minor increment in the permeability of BBB and increased oxidative stress.5 The missed R2* value difference in RN indicate the lower sensitivity of R2* map to iron content distribution in comparison with QSM. This may be because R2* map possesses poor resistance to magnetic field inhomogeneity and reflects the summation of magnetic properties from local and surrounding tissues.6Conclusion
HA hypoxia can lead to increase of brain iron concentration, especially in deep GM regions after HA acclimatization, which should raise concern over cerebral impairment due to activities in HA.Acknowledgements
This work was supported by the National Natural Science Foundation of China under Grants 11474236, U12322121, 81171331 and 81471630, the Specialized Research Fund for the Doctoral Program of Higher Education of China under Grant 20130121110014, and Science and Technology Project of Fujian Province of China under Grant 2016Y0078.References
1. Dixon S J, Stockwell B R. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 2014; 10(1): 9-17.
2. Schommer K, Kallenberg K, Lutz K, et al. Hemosiderin deposition in the brain as footprint of high-altitude cerebral edema. Neurology 2013; 81(20): 1776-1779.
3. Liu J, Liu T, de Rochefort L, et al. Morphology enabled dipole inversion for quantitative susceptibility mapping using structural consistency between the magnitude image and the susceptibility map. Neuroimage 2012; 59(3): 2560-2568.
4. Hallgren B, Sourander P. The effect of age on the non-haemin iron in the human brain. J. Neurochem. 1958; 3(1): 41-51.
5. Bailey D M, Bärtsch P, Knauth M, et al. Emerging concepts in acute mountain sickness and high-altitude cerebral edema: from the molecular to the morphological. Cell. Mol. Life Sci.2009; 66(22): 3583-3594.
6. Barbosa J H O, Santos A C, Tumas V, et al. Quantifying brain iron deposition in patients with Parkinson's disease using quantitative susceptibility mapping, R2* and R2. Magn. Reson. Imaging 2015; 33(5): 559-565.
Figures