3661
Correlations of SWI, QSM, and R2* map with neuromelanin and iron distributions from post-mortem human substantia nigra samples.1Department of Biomedical Engineering, Ulsan National Institute of Science and Technology, Ulsan, Korea, Republic of, 2Department of Electrical and Computer Engineering, Ulsan National Institute of Science and Technology, Ulsan, Korea, Republic of, 3Department of Neurology, Pusan National University Yangsan Hospital, 4Department of Anatomy, Pusan National University School of Medicine, Yangsan, Korea, Republic of
Synopsis
Spatial characterizations of neuromelanin and iron contents in human substantia nigra provide critical information in diagnosing and treating Parkinson's disease. In this work, MR investigations of susceptibility weighted imaging (SWI), R2* mapping, and quantitative susceptibility mapping (QSM) were performed with three post-mortem human substantia nigra samples at 7T and correlated with corresponding histological slides. Magnetization transfer (MT) based T1-weighted MRI technique was also conducted to validate its reputed neuromelanin sensitivity as well.
Purpose
Parkinson's disease is one of the major progressive neurodegenerative diseases associated with iron deposition and loss of dopaminergic neurons which contain neuromelanin in the substantia nigra.1 Identifying neuromelanin and iron distribution using MRI may provide a critical biomarker to monitor the evolution of Parkinson's disease. Previously, several studies have reported magnetization transfer T1 weighted neuromelanin sensitive MRI at 3T provides the neuromelanin-specific hyperintense region compared to the surrounding tissues.2 The quantification of iron content in the substantia nigra is also attractive biomarker, as iron causes a significant increase of susceptibility based contrast particularly at high field strength.3 The purpose of this study was to correlate MT T1 weighted imaging, susceptibility weighted imaging (SWI), R2* (1/T2*) mapping, and quantitative susceptibility mapping (QSM) of post-mortem human substantia nigra with corresponding histological slides containing neuromelanin and iron information.Method
Post-mortem MRI and histological validation were performed for three brains from normal individuals without any diagnosis of Parkinson's disease. The midbrains including half of the SN from all subjects were trimmed to the appropriate sizes using a customized cast for the post-mortem MR scan and fixed in the 10% neutral buffered formalin solution. The MRI experiments were conducted at 7T MRI system (Bruker, Germany). Neuromelanin sensitive MRI without and with magnetization transfer pulse were acquired with a 2D RARE sequence. Quantified R2* (1/T2*) values were calculated from 2D MGE sequence data, respectively. SWI was acquired from the postprocessing of magnitude and phase data at a specific TE of MGE. The susceptibility map was produced by quantitative susceptibility mapping algorithm (QSM). The overall MR images had common parameters: FOV = 35 × 35 (mm), matrix size = 256 × 256, thickness = 500 μm. After ex vivo MR experiments, the midbrains were sectioned to 50μm thickness according to a 500μm thick MR image. The sectioned slices were stained with three kinds of histologies; H&E staining, Perl's prussian blue staining, and luxol fast blue staining. The pixels containing iron and neuromelanin from co-registered Perl stained images were detected to quantify the contribution of iron and neuromelanin to the iron sensitive MRI techniques. The Spearman's rank correlation coefficient were calculated from iron and neuromelanin concentration with respect to corresponding R2*, SWI, and susceptibility values. The partial regression coefficient beta of neuromelanin and iron to R2* value were calculated and analyzed as a function of the iron concentration.Result
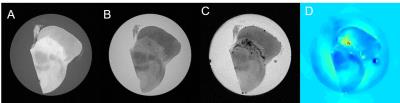
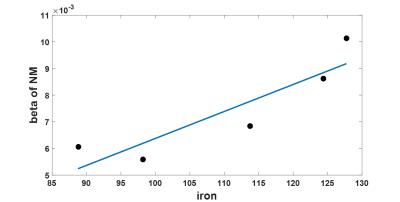
As shown in Figure 1, the significant contrast in the neuromelanin sensitive MRI was generated from magnetization transfer effect. However, the hyperintense region exactly matched with the area of segmented substantia nigra from luxol fast blue staining, but not necessarily co-localized with neuromelanin rich region. Susceptibility mismatch based MRI techniques from all subjects were highly correlated not only with iron but also with neuromelanin populations as shown in Figure 2. Iron and neuromelanin contents were observed to induce the increase of R2* and susceptibility values. The β value of neuromelanin concentration to corresponding R2* value within each sample was positively correlated with respective iron concentration as shown in Figure 3.Discussion
We observed that the MT T1-weighted MRI of post-mortem substantia nigra does not co-localize with the highly neuromelanin concentrated region, but highlights the entire substantia nigra with reducing signal intensity of the myelin of the surrounding white matter from MT effect. It is also verified that the iron and neuromelanin produce the susceptibility related MRI contrasts at 7T. We speculate that the iron is chelated to the neuromelanin and makes neuromelanin to be paramagnetic. The sensitivity of R2*, QSM and SWI to iron and neuromelanin was quite similar. The partial regression coefficients β of iron and neuromelanin to corresponding R2* values revealed that contribution of iron to R2* is similar across whole subjects. The contribution of neuromelanin to R2* is different among subject, and is positively correlated with respective iron contents in the corresponding slide. This method may provide unique way of evaluating the degree of spatial iron-chelation of neuromelanin of substantia nigra samples.Acknowledgements
This is a collaborative study between the Pusan National University Yagnsan Hospital and the Ulsan NationalInstitute of Science and Technology, South Korea. This research was supported by Research Institute forConvergence of BioMedical Science and Technology Grant (40-2013-001), Pusan National University YangsanHospital.
This work was also supported by the National Research Foundation of Korea Grants funded by the KoreanGovernment (No. 2014 R1A1A1 008255)
References
1. Sofic, E., et al. "Increased iron (III) and total iron content in post mortem substantia nigra of parkinsonian brain." Journal of neural transmission 74.3 (1988): 199-205.2..
2. Sasaki, Makoto, et al. "Neuromelanin magnetic resonance imaging of locus ceruleus and substantia nigra in Parkinson's disease." Neuroreport 17.11 (2006): 1215-1218.3.
3. Lee, Jae-Hyeok, et al. "The Neuromelanin-related T2* Contrast in Postmortem Human Substantia Nigra with 7T MRI." Scientific Reports 6 (2016).
Figures

MT T1 neuromelanin sensitive MRI
A : The slice of T1 weighted image from fast spin echo sequence without MT pulse in 7T system, B : One of the 20 slices with same geometry as A from fast spin echo sequence with MT pulse in 7T system, C : The KB staining to validate the boundary of SN