3637
Assessment of Resting State Perfusion and Coherent Large-Scale Brain Networks in Healthy Aging Using Arterial Spin Labeling Perfusion MRI1Radiology, University of Navarra Hospital, Pamplona, Spain, 2Center for Functional Neuroimaging, University of Pennsylvania, Philadelphia, United States, 3Neurology, University of Navarra Hospital, Pamplona, Spain, 4Biomedical Engineering, TECNUN, University of Navarra, San Sebastian, Spain
Synopsis
Cognitive decline is associated with aging even in the absence of disease. In this study ASL perfusion fMRI was used to investigate changes in perfusion and resting state networks connectivity due to aging, by comparing two groups of healthy subjects (young and elderly). Results showed perfusion deficits in the elderly group, in association areas, related with advanced cognitive abilities. Disruptions in varios core RSNs were also detected. Assessment of perfusion and resting functional connectivity jointly could be a good predictor of cognitive decline and a good biomarker for treatments aiming to extend cognitive abilities.
Introduction
Cognitive decline is associated with aging even in the absence of disease. It can emerge from multiple factors that affect executive function and memory. Aging related brain changes include global brain volume shrinkage, microvasculature impairment and neurovascular alterations with global cerebral flood flow (CBF) reduction. Neuroimaging techniques have been used to investigate these changes invivo (1, 2). Resting state fMRI based on BOLD contrast has been employed to assess changes in resting state brain networks (3). Arterial spin labeled (ASL) perfusion fMRI offers the possibility of measuring cerebral blood flow (CBF) and assessing resting state networks (RSNs) by means of evaluating the dynamic fluctuations in the CBF time series (4, 5).
The goal of this study was to investigate changes in cerebral perfusion and RSNs connectivity in healthy aging using ASL.
Methods
Subjects: Thirty-two elderly (age 72±6, 13M) and 31 young (24±6, 15M) healthy subjects participated in the study, after signing written informed consent. Elderly participants underwent extensive neuropsychological testing to assure that they were not suffering from abnormal cognitive decline due to an emerging neurological disorder. In the elderly group: Minimental State Examination scores were 28.7±1.6; 9 subjects suffered from hypertension and 3 from diabetes mellitus.
Scanning protocol: The study was performed on a 3T Trio using a 32-channel head-array. The scanning session included 3D-T1-weighted and T2-FLAIR sequences. ASL data were acquired with PCASL (labeling time = 1.6s, post-labeling delay = 1.5s) and background suppressed 3D GRASE (parameters: TE=29.3s, TR=3.5s, in-plane resolution=4x4 mm2, 16 slices, slice thickness=7mm, 50 label/control pairs acquired in 6 minutes). 5 control images were acquired without background suppression for calculation of CBF.
Data preprocessing (Matlab scripts and SPM12): GRASE images were realigned, coregistered to the anatomical dataset, and pairwise subtracted. Outlier images (i.e., when global perfusion differed from the mean of the perfusion time-series by ±2 standard deviations) were omitted from the mean CBF map. CBF maps were computed using the single compartment ASL perfusion model. Movement outliers were identified as images for which the pair (label/control) average head motion was > 4 mm or the difference was > 2 mm. T1-weighted images were normalized to a study-specific grey matter template. CBF mean maps and perfusion time series were then normalized to the same template and mapped into MNI space, followed by smoothing using 8 and 6 mm FWHM Gaussian kernels, respectively. FLAIR images were visually rated using the Fazekas scale (0 to 3).
CBF data analysis: Voxel-wise comparison between young and older groups of the CBF mean maps, scaled to the subject-specific whole brain mean CBF, was performed using a two-sample t-test, masked by a group mask. Areas of perfusion change were identified using p<0.05 after FWE correction at the cluster level, with a cluster-generating threshold of p-value < 0.001.
Functional connectivity. Independent component analysis:
ICA was performed using the spatial group ICA for fMRI toolbox (GroupICATv4.0a; icatb.sourceforge.net), using the Infomax algorithm. In the final stage of back-reconstruction, time courses and spatial maps were computed for each subject. Both the spatial pattern and frequency spectrum of each independent component (IC) were visually inspected to determine its relationship to a RSN and possible physiological artifacts. Individual subject perfusion IC maps were then entered into SPM12 random-effects analyses two-sample t-tests, masked with a group mask. Connectivity maps from the two groups were compared with a p-value < 0.05 after FWE cluster correction.
Results and Discussion
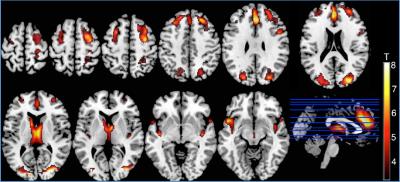
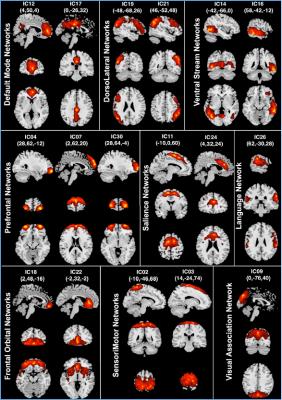
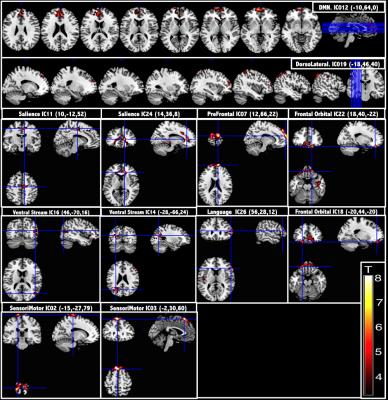
Fazekas scores in the elderly group were 0.97±0.65. Voxel-wise comparisons of relative CBF maps, revealed areas of hypoperfusion in the cortex of the older group that could not be explained just by a global CBF reduction produced by aging. Perfusion decreases were found in the frontal, occipital, insular, parietal and cingulate cortices and thalamic areas (Fig. 1). Seventeen ICs were identified as RSNs (Fig. 2). The voxel-wise comparisons of RSN connectivity maps between young and elderly groups showed that RSNs were disrupted by aging (Fig. 3). Fronto-parietal brain systems appeared to be the most affected areas. The disruption manifested itself as a decrease in connectivity that affected some vital core networks, and it could be an underlying cause of the cognitive decline associated to aging.Conclusion
This work demonstrates the feasibility of using ASL fMRI to study brain changes related to aging. Perfusion deficits were located in association areas, implicated in advanced cognitive abilities. Disruptions in varios core RSNs were detected in the older group. Assessment of perfusion and resting functional connectivity jointly could be a good predictor for cognitive decline and a good biomarker for treatments aiming to extend cognitive abilities.Acknowledgements
Grant SAF2014-56330-RReferences
1. Crivello et al., PLoS ONE 9:e114478 (2014).
2. Liu et al., Mag Reson Med 68:912-922 (2012).
3. Ferreira et al., Neurosci. Biobehav. Rev. (2013).
4. Vidorreta et al., ISMRM Perfusion Workshop, Amsterdam (2012).
5. Jann et al., Neuroimage 106:11-122 (2015).
Figures