3630
2D CAIPIRINHA improves accelerated 3D GRASE ASL1Department of Cognitive Neuroscience, Maastricht University, Maastricht, Netherlands, 2MR Application Development, Siemens Healthcare, Erlangen, Germany
Synopsis
Arterial spin labelling (ASL) is the primary non-invasive MRI approach to measure cerebral blood flow in healthy subjects and patients. Recently, a consensus paper has recommended segmented versions of 3D spin-echo readouts like GRASE, but these are susceptible to motion and have poor temporal resolution. To alleviate these drawbacks, we propose to accelerate the 3D GRASE readout and utilize 2D CAIPIRINHA for the reconstruction. We demonstrate that our approach is superior or at least equivalent to the 2D GRAPPA technique, depending on the acceleration factor used. The proposed approach will particularly benefit functional and clinical ASL applications.
Purpose
Arterial spin labelling1 (ASL) is the primary non-invasive MRI approach to measure cerebral blood flow (CBF) in healthy subjects and patients. The ASL white paper2 recommends the use of pseudo-continuous labelling, background suppression and 3D spin-echo sequences. Since T2-decay during the readout can cause significant through-plane blurring in 3D single-shot acquisitions with high spatial resolution and/or whole-brain coverage, currently the use of segmented approaches is advised. However, segmented readouts require multiple RF excitations, or shots, to acquire the complete k-space, and are thus susceptible to motion artefacts, which are particularly problematic for ASL use in the clinic. One way to reduce the duration or the number of shots per measurement is through data undersampling and reconstruction using parallel imaging techniques3,4 and RF coil arrays. Nevertheless, data undersampling typically leads to a loss in image signal-to-noise ratio (SNR) and temporal SNR (tSNR). The extent of this SNR loss will depend on the acceleration factor (AF) and the properties of the receive array, but can be reduced by utilizing controlled aliasing as in 2D CAIPIRINHA5. In this study, we explore the benefits of CAIPIRINHA for background-suppressed pCASL6,7 with accelerated 3D GRASE8 readout at 3T.Methods
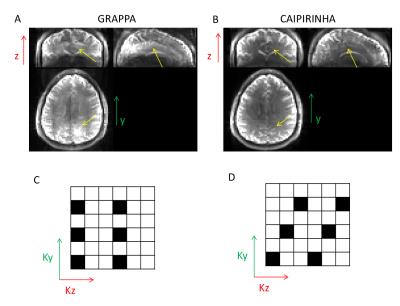
A prototype 3D GRASE pCASL sequence supporting simultaneous segmentation and GRAPPA4 acceleration, along with 2D CAIPIRINHA shifts was implemented on a MAGNETOM Prisma 3T (Siemens Healthcare, Erlangen, Germany) with a 64-channel head coil. The CAIPIRINHA shifts were realized by either modulating the EPI readout with Δkz-blips (for phase-encoding AF ≤ partition-encoding AF) or by shifting successive kz-planes by Δky and appropriate echo shifts (for phase-encoding AF > partition-encoding AF). Experiments were performed in seven healthy volunteers after obtaining informed consent. Optimized background suppression9 was applied. A Time-Of-Flight angiogram was acquired to determine the position of the labelling plane, and a subject-specific grey-matter (GM) mask was generated from a T1-weighted MPRAGE scan. Six 4-minute ASL protocols were performed in pseudo-randomized order, each with different combinations of in-plane (phase-encoding) and through-plane (partition-encoding) acceleration factors [AF_PE x AF_Pa (CAIPIRINHA_shift)], utilizing either GRAPPA or CAIPIRINHA reconstruction. The accelerated protocols were either 2-shot segmented [2x1 (y1), 1x2 (z1), 3x1 (y1), 1x3 (z2)] or single-shot [2x2 (z1), 2x3 (z1)]. For comparison, a scan without parallel acceleration (4 shots) was also obtained. Additional control images without background suppression and long TR were acquired immediately after each protocol for CBF quantification. All scans had 3mm isotropic nominal voxel size, labelling duration/post-labelling delay/TR = 1500/1580/4120ms and 30 or 32 slices depending on the AF_Pa. The data were motion-corrected and coregistered with SPM8. Voxel-wise CBF was computed according to Vidoretta et al.9, whereas perfusion tSNR maps and perfusion tSNR efficiencies – according to Li et al.10.Results
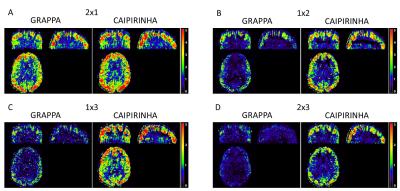
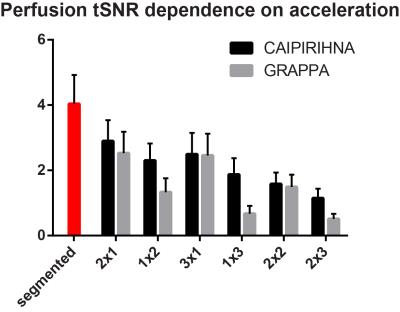
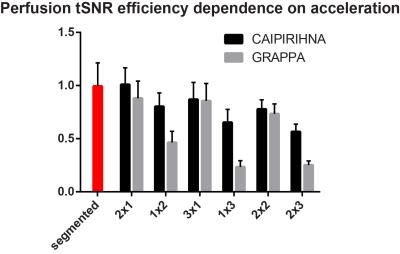
Figure 1 demonstrates that acceleration in the partition-encoding direction can result in residual aliasing artefacts when CAIPIRINHA is not used. However, no significant differences in mean GM CBF were found among all the protocols tested once regions with artefacts were excluded. Increasing the AF decreases the perfusion tSNR, and for acquisitions with CAIPIRINHA the reduction is smaller or equal to the reduction when using GRAPPA (Figures 2 and 3). CAIPIRINHA has significantly higher perfusion tSNR than GRAPPA for the 1x2, 1x3 and 2x3 accelerations. Despite having significantly lower perfusion tSNR than the segmented acquisition, the tSNR efficiencies of the accelerated acquisitions compare more favourably to it (Figure 4).Discussion
The artefact reduction and perfusion tSNR increases with CAIPIRINHA compared to GRAPPA allow higher acceleration factors, and therefore alleviate the need for readout segmentation, which in addition reduces the motion sensitivity of 3D GRASE ASL. Furthermore, the higher acceleration factors can be employed to shorten the duration of the GRASE readout reducing image blurring and distortions in susceptibility-affected regions, thereby resulting in higher effective resolution. Alternatively, the higher acceleration factors can also be used to increase the nominal spatial resolution. All of these advantages apply equally to pulsed 3D GRASE ASL since they do not depend on the labelling scheme.
The aforementioned scenarios offer significant immediate benefits for functional and clinical ASL – two areas where the non-invasiveness of ASL is particularly valuable, but the current segmented approaches are performing suboptimally. Recently, acceleration techniques for other commonly-used ASL readouts like stack-of-spirals have been proposed11. The advantage of Cartesian CAIPIRINHA sampling and reconstruction is that it can be readily implemented on the scanner and the data evaluated online, which facilitates its direct clinical use.
In conclusion, CAIPIRINHA-accelerated 3D GRASE improves on several shortcomings of current readout approaches, advancing ASL closer to its full potential.
Acknowledgements
No acknowledgement found.References
1 Detre et al. MRM 1992. 23:37-45;
2 Alsop et al. MRM 2015. 73:102-116;
3 Pruessmann et al. MRM 1999. 42:952-962;
4 Griswold et al. MRM 2002. 47:1202-1210;
5 Breuer et al. MRM 2006. 55:549-556;
6 Dai et al. MRM 2008. 60:1488-1497;
7 Wu et al. MRM 2007. 58:1020-1027;
8 Oshio and Feinberg. MRM 1991. 20:344-349;
9 Vidorreta et al. NMR Biomed 2014. 27:1387-1396;
10 Li et al. Neuroimage 2015. 106:170-181;
11 Chang et al. MRM in press. doi: 10.1002/mrm.26549
Figures