3621
The reproducibility of absolute ASL-CBF: assessing the stability of absolute CBF, M0 and calibration images1Fraunhofer MEVIS, Bremen, Germany, 2Universitaet Bremen, Bremen, Germany
Synopsis
In this abstract the reproducibility of absolute CBF as well as the M0- and an optional calibration-image is assessed during individual scanning-sessions and also over the entire day. Comparably high reproducibility was found for all three types of images, which agrees with similar earlier findings. However, also the uncertainty of the corresponding fits was found to be high.
PURPOSE
Arterial spin labeling (ASL) is a non-invasive method for the measurement of cerebral blood flow (CBF). A clinically important feature of ASL is the possibility to quantify perfusion. To compute quantitative (absolute) CBF additional maps like an M0- and a calibration-image are necessary1. To establish absolute CBF as a clinical imaging-biomarker it is important to assess its reproducibility. However, this also depends on the reproducibility of the M0- and the optional calibration-image. Therefore, in this study the reproducibility of absolute CBF, M0- and calibration-images is determined during an individual session and during the day.METHODS
Five healthy volunteers (22.4±2.4 years, 4 female) underwent three MR scanning-sessions (3T-Skyra, SIEMENS Healthcare, Germany) within the same day (morning, noon, evening). Each session was divided into three blocks. Each block comprised the acquisition of WH-pCASL2 images, an M0- and a calibration-image (see figure 1).
Imaging:
- WH–pCASL: 8x7 Walsh-ordered Hadamard-matrix, subbolus duration: 450 ms, post labeling delay: 300 ms, two background-suppression (BS) pulses
- 3D-GRASE3 image-readout: TR/TE=6000/18 ms, slices=24, matrix= 64x48, FOV=192x256 mm²
- M0-image: same 3D-GRASE readout, however no BS, TR=10000 ms
- calibration-image: same as M0-image but using body-coil instead of head-coil
Post-Processing:
All images of a subject were corrected for motion and realigned (3D-rigid) using MeVisLab (MeVis Medical Solutions, Bremen, Germany). Using a T1-weighted anatomical image gray matter (GM)-, white matter (WM)- and cerebro-spinal fluid (CSF)-masks were generated and registered to the ASL-images. From the WH-pCASL images multi-PLD perfusion-weighted images were decoded.
FSL-BASIL and asl_calib4,5 were then used to generate absolute CBF-maps from the multi-PLD images and the M0- and calibration-image.
To assess their reproducibility the following signals were determined for all sessions and blocks:
- mean GM- and WM-signal in absolute CBF-maps
- mean CSF-signal in M0-images
- mean whole-brain (WB) signal in calibration-image
FSL-BASIL additionally yields the voxel-by-voxel variance of CBF for each fit. From this variance-map the mean standard deviation (STD) over GM and WM was computed to estimate the uncertainty of the mean GM- and WM-signal. Finally, a total mean STD, σtot, was determined by averaging the STDs over all measurements and subjects.
To assess the stability of the M0- and calibration-signal during individual sessions, average mean CSF- and WB-signals were computed for block 1, 2 and 3. To this end signals from the morning, noon and evening sessions were averaged for each block. The errors were determined by the corresponding STD.
RESULTS
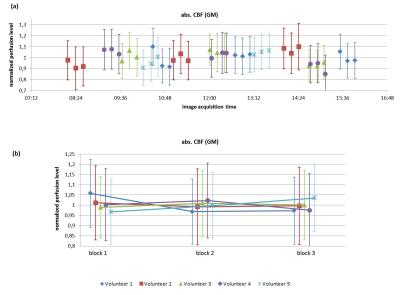
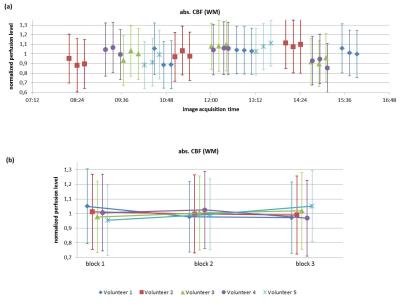
Figure 2 and 3 show absolute CBF-values in GM and WM plotted over time of day (a) and blocks of a session (b). For better comparability the CBF-values of each subject were normalized to her/his diurnal perfusion-level, i.e. her/his CBF-value averaged over all sessions and blocks. In both figures the variation of mean CBF-values lies within the 1σ-uncertainty range of the fit.
The observed total mean STD for GM and WM are σtot(GM)=7.34 ml/100g/min (~17%) and σtot(WM)=6.34 ml/100g/min (~25%).
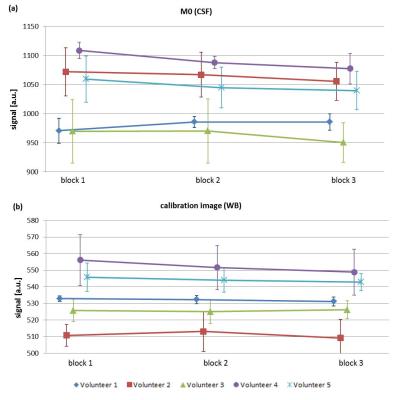
Figure 4 shows the CSF-signal in the M0-image and the WB-signal in the calibration-image. Again, the variation of the signals stays within the 1σ-uncertainty range for both images.
DISCUSSION
The results show that at the given accuracy of the fit CBF is over the day a fairly constant parameter. This confirms earlier results by Parkes et al.6, however using a larger sample (N=5). We also investigated perfusion changes during individual scanning sessions and found also here that CBF remains constant.
However, the 1σ-uncertainty of the fit is comparably large, as the GM- and WM-values for σtot show. This implies that absolute CBF-values have to be assessed carefully. Further investigation of the reproducibility of CBF using larger numbers of subjects is highly warranted.
Also M0- and calibration-signals remained sufficiently constant during a scanning session. Thus, in scanning sessions comprising several ASL-measurements it is possible to acquire only one M0- and calibration-image for later quantification, which eventually saves scanning-time. This is of particular importance for clinical protocols where time is a crucial factor.
This study may be limited by using young healthy volunteers only. However, in pathologies like cerebrovascular disease blood supply is impaired which can strongly affect the stability of perfusion. Future studies should therefore be focused on clinical datasets.
CONCLUSION
This study assessed the reproducibility of CBF as well as of M0- and calibration-signals. All three were found to be constant within the level of uncertainty. Thus, ASL-CBF assessment appears to be independent of the specific timing of scans within sessions and during the day. This increases the flexibility in the setup of studies, which use ASL-CBF as imaging-biomarker and is an important step toward s the use of absolute ASL-CBF for clinical applications.Acknowledgements
No acknowledgement found.References
1. Alsop DC, Detre JA, Golay X, et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn. Reson. Med. 2015;73:102–116. doi: DOI: 10.1002/mrm.25197.
2. von Samson-Himmelstjerna F, Madai VI, Sobesky J, Günther M. Walsh-ordered Hadamard time-encoded pseudocontinuous ASL (WH-pCASL). Magn. Reson. Med. 2015. doi: 10.1002/mrm.26078.
3. Günther M, Oshio K, Feinberg DA. Single-shot 3D imaging techniques improve arterial spin labeling perfusion measurements. Magn. Reson. Med. 2005;54:491–498. doi: 10.1002/mrm.20580.
4. Jenkinson M, Beckmann CF, Behrens TEJJ, Woolrich MW, Smith SM. Fsl. Neuroimage 2012;62:782–90. doi: 10.1016/j.neuroimage.2011.09.015.
5. Chappell MA, Groves AR, Whitcher B, Woolrich MW. Variational Bayesian Inference for a Nonlinear Forward Model. IEEE Trans. Signal Process. 2009;57:223–236. doi: 10.1109/TSP.2008.2005752.
6. Parkes LM. Quantification of cerebral perfusion using arterial spin labeling: Two-compartment models. J. Magn. Reson. imaging 2005;22:732–736. doi: 10.1002/jmri.20456.
Figures