3617
Improved spatial encoding for vessel-selective pCASL: improving efficiency, minimising mis-labeling, and shortening scan-time for artery specific MRA1C.J.Gorter Center for High Field MRI, Department of Radiology, Leiden University Medical Center, Leiden, Netherlands, 2FMRIB Centre, Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, United Kingdom
Synopsis
Hadamard-encoded vessel-encoded pCASL is a vessel-selective ASL technique that enables SNR-efficient vascular territory mapping for perfusion MRI. For vessel-selective ASL-MRA, however, minimising the number of encodings is necessary to achieve clinically feasible scan times. The spatial modulation of inversion in ve-pCASL is gradual, which could potentially reduce SNR-efficiency and cause mislabeling of arteries when reducing the number of encodings. In this study, the modulation of ve-pCASL was optimized to achieve sharper inversion to avoid signal contamination of non-targeted arteries and achieve 4D-MRA with a minimal number of Hadamard-encodings.
Purpose
Vessel-selective arterial spin labeling (ASL) enables the visualization of blood flow arising from individual arteries, which is an important advantage of ASL-MRA over contrast-enhanced MRA. In vessel-encoded pseudo-continuous ASL (ve-pCASL) perfusion MRI1, SNR-efficient territorial separation can be achieved by employing Hadamard-encodings across the feeding arteries whilst repeating measurements for averaging. For ASL-MRA, however, 3D data is usually acquired in a multi-shot fashion and the entire scan-time is used for spatial encoding, rather than averaging, to achieve high spatial resolution. Therefore, the number of vessel-encodings will increase scan-time proportionally. In a previous report, eight Hadamard-encodings of four arteries resulted in a scan-time of 18 minutes2. The three-vessel encoding scheme of Günther3 only requires four Hadamard-encodings, in which each of right internal carotid artery (RICA), left ICA (LICA) and both vertebral arteries (VAs) are labeled in different combinations, along with a non-selective control image. This scheme would reduce scan-time by a factor of two. However, the pulsed-ASL approach of Günther suffers from difficult planning of the selective labeling slab to avoid contamination from non-targeted arteries, because it requires large coverage of tortuous vessels in the inferior-superior direction. Although ve-pCASL avoids this problem, it requires complicated post-processing such as the Bayesian framework5 because the inversion modulation of ve-pCASL is more gradual than pulsed-ASL. The purpose of this study is to achieve a sharper ve-pCASL spatial modulation to improve labeling efficiency and minimize partial labeling of nearby arteries, enabling simpler post-processing for 4D-MRA within a clinically feasible scan-time.Theory
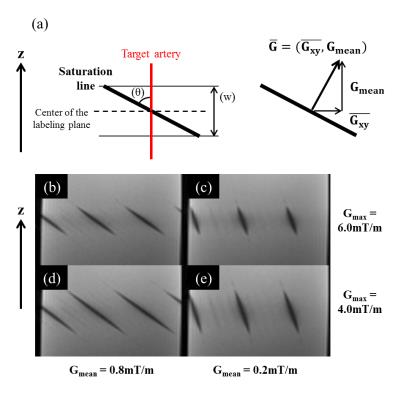
Ve-pCASL has two different approaches to achieve the spatial modulation of inversion: the bipolar approach in which the transverse gradient (Gxy) is applied alternately positive and negative, and the unipolar approach in which Gxy is not alternated. Magnetization is inverted at the positions where the phase of the labeling pulses is aligned with the phase shift produced by Gxy. Because of the pCASL mean gradient (Gmean) in z-direction, this in-phase position varies with z. With bipolar Gxy, the phase coherence away from the middle of the labeling plane is lost, but in the unipolar approach, the consistent Gxy and RF phase cycling result in phase coherence within tilted planes, visible as lines of saturation in a static phantom (Figure-1b-e). The effective inversion width (“w” in Figure-1a) depends on the maximum gradient of pCASL (Gmax), whereas Gmean determines the angle of the saturation line (“θ” in Figure-1a). Accordingly, the inversion modulation of the unipolar approach is dependent on Gmax and Gmean.Methods
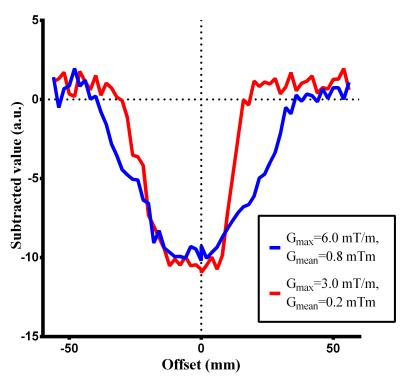
As Wong et.al. suggested4, the unipolar approach is superior to the bipolar approach, therefore all experiments were performed using unipolar gradients. By Bloch equation simulation, ve-pCASL inversion modulation was simulated with Gmax of 6 mT/m and Gmean of 0.8 mT/m (default settings, as per Wong et al.1,4) and with Gmax and Gmean decreased in several steps to 2mT/m and 0.2 mT/m, respectively. Next, in-vivo studies were performed in two healthy volunteers to validate the simulation results: (1) Perfusion images were acquired whilst offsetting a left-right encoding in small increments relative to the RICA and LICA locations; averaged perfusion signal was extracted from LICA/RICA masks generated by a conventional ve-pCASL perfusion image with eight Hadamard-encodings. (2) ve-pCASL 4D-MRA was acquired using the three-vessel encoding scheme using the optimized settings of Gmax and Gmean, with scan-time 7min12s. All scans were performed on a Siemens 3T Verio under a technical development protocol agreed by local ethics and institutional committees.Results and discussion
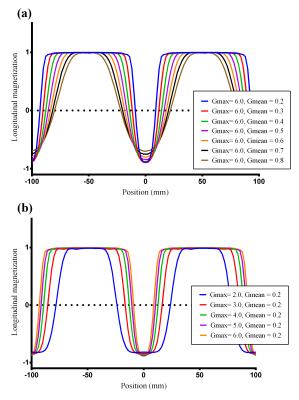
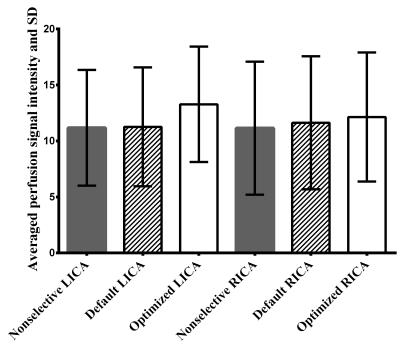
Simulated inversion modulations showed sharper selectivity for lower Gmean (Figure-2a), which is attributed to steeper saturation line (Figure-1c/e). For a fixed Gmean, lower Gmax produced broader inversion width (Figure-2b), which is explained by wider effective inversion width (Figure-1d/e) which produced concomitant broadening of the inversion width. Figure-3 shows the averaged perfusion signal curves as a function of the selective labeling offset measured in-vivo. With optimized Gmean and Gmax sharper selectivity was achieved, confirming the simulation results. Comparison of the perfusion signal at offset=0 (Figure-4) did not suggest any differences in the labeling efficiency between the default and optimized settings. Figure-5 shows ve-pCASL 4D-MRA of a healthy volunteer acquired with the three-vessel encoding scheme and the optimized settings. By optimization, labeling of each RICA, LICA and VAs were achieved with high selectivity, and no obvious mislabeling was observed. Compared to the previous report using eight Hamadard-encodings, scan-time was halved.Conclusion
In this study, we presented an approach to improve the inversion modulation in ve-pCASL, enabling faster scan-times. Controlling the inversion modulation with Gmax and Gmean could be beneficial, not only for 4D-MRA, but also for perfusion imaging.Acknowledgements
This research was supported by the EU under the Horizon2020 program (project: CDS-QUAMRI).References
1. Wong EC. Vessel-Encoded Arterial Spin-Labeling UsingPseudocontinuous Tagging. Magn Reson Med (2007) 58:1086-91.
2. Okell TW, Schmitt P, Bic X, Chappell MA, Tijssen RHN, Sheerin F, Miller KL and Jezzard P. Optimization of 4D vessel-selective arterial spinlabeling angiography using balanced steadystatefree precession and vessel-encoding. NMR in Biomed (2016) 29:776-86
3. Guenther M. Efficient Visualization of Vascular Territories in theHuman Brain by Cycled Arterial Spin Labeling MRI. Magn Reson Med (2006) 55:671-5.
4. Wong EC, Guo J. Blind detection of vascular sources and territories using randomvessel encoded arterial spin labeling. Magn Reson Mater Phy (2012) 25:95–101
5. Chappell MA, Okell TW, Payne SJ, Jezzard P, Woolrich MW. A fast analysis method for non-invasive imaging of blood flow in individual cerebral arteries using vessel-encoded arterial spin labelling angiography. Med. Image Anal (2012) 16:831-9
Figures