3616
Solving the dark-sides of multiband-ASL: A framework to correct for increased motion artefacts in MB-ASL due to sharp transitions in the level of background suppression1C.J.Gorter Center for High Field MRI, Department of Radiology, Leiden University Medical Center, Leiden, Netherlands, 2FMRIB Centre, Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, United Kingdom, 3Institute of Biomedical Engineering, University of Oxford, Oxford, United Kingdom
Synopsis
Recently, incorporation of multi-band (MB-) EPI into ASL has been reported, enabling increased spatial coverage without compromising SNR of distal slices due to longer post-labeling delay time. However, the combination of MB-EPI and ASL with the background suppression (BGS) could potentially induce problems when motion correction (MoCo) is required. In this study, we demonstrate that subtraction artefacts can be introduced when performing MoCo of MB-BGS-pCASL and that these artefacts can degrade image quality considerably. We propose a new framework that corrects for image degradation caused by MoCo of MB-BGS-pCASL data, thereby greatly improving robustness and thus usefulness of MB-BGS-pCASL.
Purpose
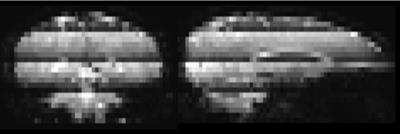
Simultaneous multi-slice (SMS, a.k.a. multiband -MB-) EPI1, excites multiple slices at the same time, thereby reducing the number of excitations per TR. This approach has been proven to be very advantageous for fMRI and DTI2,3. Recently, incorporation of MB into ASL has been reported4,5, enabling increased spatial coverage without compromising SNR of distal slices due to longer post-labeling delay times. Unlike fMRI or DTI, ASL employs background suppression (BGS) to decrease physiological noise and motion artefacts from background tissue. However, the combination of MB and BGS could potentially induce problems when motion correction (MoCo) is required: due to the MB-excitation, slices with the most effective BGS are adjacent to slices which experience the lowest degree of BGS, resulting in discrete dark lines (clearly visible on sagittal and coronal reformatted images as shown in Figure-1). When motion occurs in the slice direction (i.e. rotation about the x- or y-axis or translation in z-direction), tissue can have experienced a different level of BGS during the control than during the label condition. After MoCo has successfully realigned the images, severe subtraction errors will occur due to these differences in BGS-level. The purpose of this work is to present a new framework for MoCo for MB-BGS-pCASL imaging, which clearly separates the perfusion signal from signal contamination due to different BGS-levels after MoCo realignment.Theory
The proposed framework consists of two steps: (1) BGS homogenization to remove the background tissue signal intensity difference over slices, which will reduce MoCo-induced subtraction errors. Homogenization is achieved by applying a BGS homogenization factor per slice as obtained from the ratio of the mean tissue value of the M0 and the MB-BGS-pCASL scan. (2) Removal of residual errors after MoCo using a general linear model (GLM): y=[xperfxcontami][βperfβcontami]+c, where y is the motion corrected pCASL time-series at a certain voxel, xperf is the labelling paradigm [0.5, -0.5, …, 0.5, -0.5]T multiplied by the BS correction factor to correct for the unwanted scaling of the perfusion signal during the homogenization step, xcontami represents artefactual signal changes induced by MoCo, obtained from subtracting the ASL-datasets before and after MoCo, βperf and βcontami are fitting coefficients for xperf and xcontami, respectively, and c is the mean static tissue signal.Methods
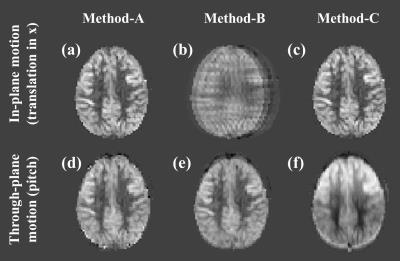
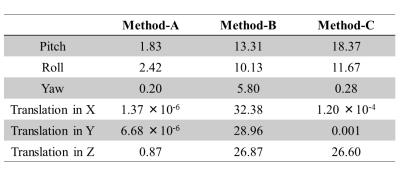
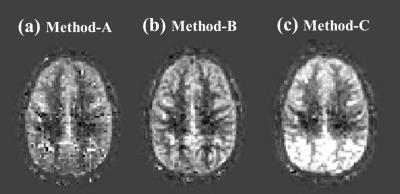
Simulation: On a pCASL perfusion, M0 and T1-map acquired from a volunteer, artificial motion (translation in the x/y/z-direction and rotation around the x/y/z-axis) was applied. Using these data, control images were obtained by calculating the effect of the saturation and BGS-inversion pulses on the M0-magnetization while taking into account the slice-timing. Label images were obtained similarly, but additionally the pCASL-signal was subtracted. These data underwent the processing described above and the perfusion image was generated with GLM (Method-A). For comparison, results generated with a simple subtraction without MoCo (Method-B) and with MoCo but without BS correction (Method-C) are shown. To quantify subtraction errors, similar dataset without perfusion signal (all control images) was generated and the variance of the subtracted image was calculated.
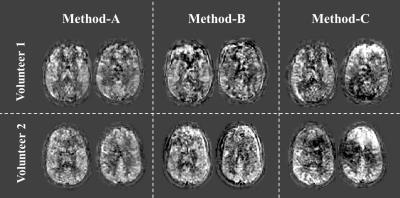
In-vivo study: In-vivo studies were performed in four healthy volunteers on a 3.0T scanner (Ingenia, Philips) with MB factor of 3. Volunteers were instructed to move their head during scans. These data were processed by methods A, B and C. This study was approved by local IRB and volunteers provided informed consent.
Results and discussion
Figure-2 shows simulation images of in-plane motion (a-c) and pitch (d-f). Whereas MoCo improved image quality for in-plane translation (2c showing the importance of MoCo for this type of motion), for pitch it resulted in severe subtraction artefacts (2f), mainly seen as a gradient in signal intensity. Interestingly, image quality without MoCo (2e) was relatively spared for pitch motion. By applying our framework (Method-A), subtraction errors introduced by MoCo decreased significantly (2d), as also seen in a significant decrease in variance of the error maps (Figure-3). Figure-4 shows the results of the in-vivo studies. In most cases, Method-A improved the image quality compared to both Method-B and -C. However, in a subject with only subtle motion (Figure-5), Method-B resulted in the best image quality, which can be explained that the GLM process could to some extent also introduce additional noise.Conclusion
In this study, we demonstrated that subtraction artefacts can be introduced when performing motion-correction of MB-BGS-pCASL data and that these artefacts can degrade image quality considerably. The proposed framework corrects for image degradation caused by MoCo of MB-BGS-pCASL data, thereby greatly improving robustness and thus usefulness of MB-BGS-pCASL, which will especially be beneficial when applied in restless subjects, and thus for clinical applications of MB-BGS-pCASL.Acknowledgements
This research was supported by the EU under the Horizon2020 program (project: CDS-QUAMRI).References
1. Moeller S, Yacoub E, Olman CA, Auerbach E, Strupp J, Harel N, Ugurbil K. Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Mag Reson Med (2010) 63:1144-53.
2. Feinberg DA, Moeller S, Smith SM, Auerbach E, Ramanna S, Gunther M, Glasser MF, Miller KL, Ugurbil K, Yacoub E. Multiplexed echo planar imaging for sub-second whole brain FMRI and fast diffusion imaging. PLoS One (2010) 5:e15710.
3. Setsompop K, Cohen-Adad J, Gagoski BA, Raij T, Yendiki A, Keil B, Wedeen VJ, Wald LL. Improving diffusion MRI using simultaneous multi-slice echo planar imaging. Neuroimage (2012) 63:569–80.
4. Feinberg DA, Beckett A, and Chen L. Arterial Spin Labeling with Simultaneous Multi-Slice Echo Planar Imaging. Magn Reson Med (2013) 70:1500-6.
5. Kim T, Shin W, Zhao T, Beall EB, Lowe MJ, Bae KT. Whole Brain Perfusion Measurements Using Arterial Spin Labeling with Multiband Acquisition. Magn Reson Med (2013) 70:1653-61.
Figures