3615
3D Whole-Brain Mapping of Cerebral Blood Flow using Velocity-Selective Pulse Trains: Evaluating Various Strategies for Background Suppression1Johns Hopkins University, Baltimore, MD, United States, 2Kennedy Krieger Institute, Baltimore, MD, United States
Synopsis
Velocity-selective arterial spin labeling (VSASL) has only been realized with 2D multi-slice acquisition. ASL with 3D readout is preferred for clinical applications and background suppression technique is essential for successful implementation of segmented 3D ASL. In this study, various strategies for background suppression are evaluated for 3D VSASL which are labeled with conventional T2prep VS pulse train, and new Fourier transform based velocity-selective saturation and inversion pulse trains, respectively. The optimal 3D whole-brain VSASL protocol is compared with PCASL for mapping cerebral blood flow on normal volunteers at 3T.
INTRODUCTION
For measurement of CBF using ASL approach, 3D readout is preferred over 2D acquisition. Background suppression (BGS) 1 is recommended in standardized protocol of PCASL using segmented 3D readout for clinical applications 2. Velocity-selective (VS) ASL 3 has the advantage of much reduced sensitivity to arterial transit time on patients with arterial occlusive diseases, e.g. stroke. However, VSASL has only been demonstrated with 2D acquisition 3-9, which limits its widespread utilization. Recently, Fourier-transform based velocity-selective saturation (VSS) and inversion (VSI) pulse trains have been introduced for MR angiography 10 and ASL 9, respectively. This is in addition to the conventional T2prep VS module 3-8. In this study, various strategies for BGS are evaluated for 3D VSASL labeled with T2prep, VSS and VSI pulse trains. The optimal 3D whole-brain VSASL protocol for CBF mapping is compared with PCASL.Methods
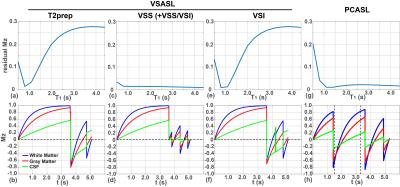
The diagram of the 3D VSASL is plotted in Fig. 1a. A global saturation 11 was applied at the beginning with a delay of 3.6 s before the label/control VS modules. Different VS modules were tested: a 20 ms T2prep pulse train (Fig. 1b); a 56 ms FT based VSS or VSI pulse train 9 (Fig. 1c). For the FT based VS modules, VSS, VSI, VSS+VSS, and VSS+VSI are used respectively (Vc=3 cm/s).
During the 1.5 s post-labeling delay (PLD), two or three non-selective inversion pulses (BGS) were applied to suppress the static tissues. The timing of the BGS pulses were tailored through a home-made iterative minimization algorithm in Matlab, to have residual signal intensities of 1% - 30% for T1 values from 0.5 s to 4.4 s. Note that the effect of VS pulse train on tissues is taken into account in the timing calculation of the BS pulses.
Experiments were performed on a 3T Philips scanner using a 32-channel head coil for reception. Different VS modules with BSG pulses were tested among 3 healthy volunteers. The VSASL protocol with the best performance was evaluated on 11 healthy subjects, and compared with PCASL protocol (labeling duration: 1.8 s, PLD of 2.0 s) with its BGS pulses.
For all ASL protocols, at the end of the PLD, a T2prep with crushing gradients were applied to suppress large-vessel signals (Vc=3 cm/s). Following a fat-suppression module, a 3D GRASE acquisition scheme was employed with FOV of 220x220x116 mm3 and acquisition resolution=3.9x4.2x4.0 mm3 (echo train duration: 121 ms, SENSE=2). With a TR=5.8 s, total measurement time with 4 averages was 5 min.
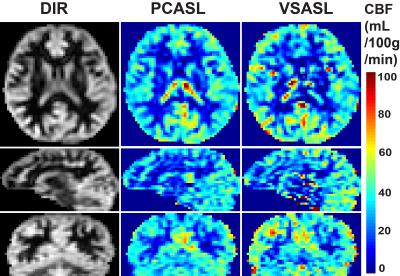
In addition, proton density-weighted image (TR=10 s) was acquired for CBF quantification purposes and a double inversion recovery (DIR) image to visualize gray matter only. Standard equations were used for CBF quantification of both the VSASL and PCASL scans 2,3.
Results and Discussion
For T2prep VS, BGS = [0.04, 1.14] s following labeling pulse; For FT based VSS, VSS+VSS, or VSS+VSI configurations, BGS = [0.2, 0.78, 1.3] s after labeling; For FT-VSI, BGS = [0.6, 0.64, 1.14] s after labeling. Fig. 2 shows the simulated residual Mz of tissues with different T1s for these VS modules (1st row) and the magnetization of tissues following corresponding VS and BGS pulses. For T2prep and VSI modules, tissues with long T1s are not well suppressed. BSG is best performed with the modules started with VSS.
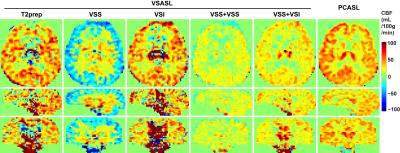
Fig. 3 shows the CBF maps using different pulse configurations, with all labeling efficiency assumed to be 1. Artifact contamination is obvious in T2prep and VSI modules. The negative CBF artifacts observed in the results using a single VSS is related to the sensitivity of excitation pulses in the VSS pulse train to B1 inhomogeneities. Results of VSS+VSS and VSS+VSI modules showed least artifacts with higher SNR in VSS+VSI. PCASL results are for comparison.
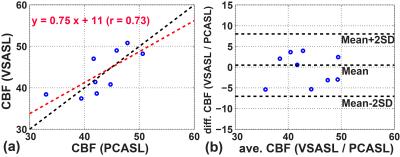
By referencing the averaged gray matter signal differences of the VSASL scan to those in the PCASL scans, the labeling efficiency of the VSS+VSI were estimated to be 0.36+/-0.06 (N=9). Note that this is lower than 0.57 reported for the single VSI module in a 2D study 9, primarily due to the additional VSS module used here for the 3D readout. The CBF maps estimated using PCASL and VSS+VSI prepared VSASL scans from one subject are shown in Fig. 4. Excellent correlation and agreement between the two methods are found with a correlation coefficient of 0.78 (Fig. 5).
CONCLUSION
The feasibility of VSASL for CBF mapping with a 3D whole-brain coverage is demonstrated after evaluating different configurations of VS modules and BGS. The VSS+VSI prepared VSASL scans showed reasonable agreement in CBF quantification with the standard PCASL method.Acknowledgements
No acknowledgement found.References
1. Ye FQ, Frank JA, Weinberger DR, McLaughlin AC. Noise reduction in 3D perfusion imaging by attenuating the static signal in arterial spin tagging (ASSIST). Magn Reson Med 2000;44(1):92-100.
2. Alsop DC, Detre JA, Golay X, Gunther M, Hendrikse J, Hernandez-Garcia L, Lu H, Macintosh BJ, Parkes LM, Smits M, van Osch MJ, Wang DJ, Wong EC, Zaharchuk G. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med 2015;73:102-116.
3. Wong EC, Cronin M, Wu WC, Inglis B, Frank LR, Liu TT. Velocity-selective arterial spin labeling. Magn Reson Med 2006;55(6):1334-1341.
4. Duhamel G, de Bazelaire C, Alsop DC. Evaluation of systematic quantification errors in velocity-selective arterial spin labeling of the brain. Magn Reson Med 2003;50(1):145-153.
5. Wu WC, Wong EC. Intravascular effect in velocity-selective arterial spin labeling: the choice of inflow time and cutoff velocity. Neuroimage 2006;32(1):122-128.
6. Qiu D, Straka M, Zun Z, Bammer R, Moseley ME, Zaharchuk G. CBF measurements using multidelay pseudocontinuous and velocity-selective arterial spin labeling in patients with long arterial transit delays: Comparison with xenon CT CBF. Journal of magnetic resonance imaging : JMRI 2012;36(1):110-119.
7. Meakin JA, Jezzard P. An optimized velocity selective arterial spin labeling module with reduced eddy current sensitivity for improved perfusion quantification. Magn Reson Med 2013;69(3):832-838.
8. Guo J, Meakin JA, Jezzard P, Wong EC. An optimized design to reduce eddy current sensitivity in velocity-selective arterial spin labeling using symmetric BIR-8 pulses. Magn Reson Med 2015;73(3):1085-1094.
9. Qin Q, van Zijl PC. Velocity-selective-inversion prepared arterial spin labeling. Magn Reson Med 2016;76(4):1136-1148.
10. Qin Q, Shin T, Schar M, Guo H, Chen H, Qiao Y. Velocity-selective magnetization-prepared non-contrast-enhanced cerebral MR angiography at 3 Tesla: Improved immunity to B0/B1 inhomogeneity. Magn Reson Med 2016;75(3):1232-1241.
11. Sung K, Nayak KS. Design and use of tailored hard-pulse trains for uniformed saturation of myocardium at 3 Tesla. Magn Reson Med 2008;60(4):997-1002.
Figures