3613
Regional Analysis of Hyperpolarized Lactate-to-Pyruvate Ratio Can Improve Sensitivity to Monitor Progression of Acute Pulmonary inflammation1Radiology, University of Pennsylvania, Philadelphia, PA, United States, 2Anesthesiology and Critical Care, University of Pennsylvania, Philadelphia, PA, United States
Synopsis
In this hyperpolarized pyruvate imaging study of acute lung injury, we assessed alterations of regional lactate-to-pyruvate ratio during the progression of lung inflammation caused by acid aspiration. The study shows that posterior lactate-to-pyruvate ratio changes more significantly after injury compared to the anterior ratio. This is consistent with the pattern observed with proton MRI. We report good correlation between increased lactate-to-pyruvate ratio due to inflammation and increased proton image intensity as a result of formation of edema, especially in the posterior regions.
Introduction
Several imaging modalities have been used to examine structural and functional changes to the lung after acute injury [1-2]. While these approaches are very useful, they examine the secondary effects of inflammation rather than targeting the underlying changes in cellularity. Recently, hyperpolarized pyruvate has shown great promise for studying the underlying alterations in metabolism and pH caused by acute lung inflammation [3-5]. However, no studies have assessed the spatial heterogeneity of metabolic alterations in the presence of global acute lung injury. We hypothesize that assessment of regional information can provide better insight into patient care and management of acute inflammation. In this study, we demonstrate that hyperpolarized pyruvate imaging can be used to assess regional changes of metabolism during the progression of experimental acid aspiration lung injury.Methods
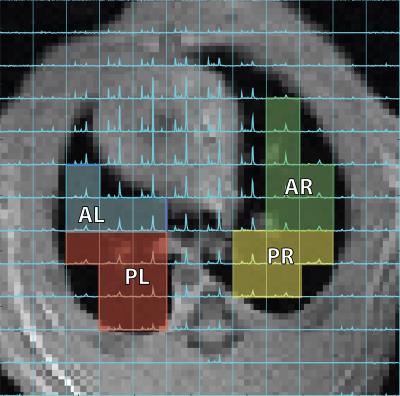
Eighteen Sprague-Dawley rats (335±34g) were ventilated with a tidal volume (TV) of 7.9±0.7 mL/kg and a positive end-expiratory pressure (PEEP) of 7.1±2.1cmH2O for 5hrs. Injured rats received doses of HP [1-13C]-pyruvate at t=60min, t=120 and t=300min after the start of ventilation. Hydrochloric acid (pH 1.25) was instilled into the trachea of the rats at t=70min at varying dosages (0.5ml/kg (n=2), 1ml/kg(n=11) and 2ml/kg (n=5)) to achieve different severity of injury between cohorts. Dynamic pulmonary compliance was measured by dividing peak-inspiratory pressure (PIP) by TV in the absence of PEEP [1], and blood oxygen level was monitored using a pulse oximeter. All rats were imaged in the supine position using a 1H/13C quadrature birdcage coil (m2m) at 4.7T (Varian Inc.). Proton images were acquired using a multi-slice gradient echo (TR/TE=80/1.5ms, α=10°, 128x128 voxels). ~22 µL of [1-13C]-pyruvate was polarized using a HyperSense DNP polarizer (Oxford Instruments) for over 1hr and was subsequently melted using 4mL of a dissolution buffer (40mM Trizma, 160mM NaOH, 50mM NaCl, 0.1g/L EDTA) at 180°C to yield a neutral isotonic solution of 80mM [1-13C]-pyruvate at ~37°C. 12s after the dissolution, hyperpolarized pyruvate was administered via the tail-vein within 8s at 5.7±2.0ml/kg dose. An axial carbon-13 chemical shift image was acquired 12s after the end of injection using a 16x16 FID-CSI sequence (TR/TE=35/0.35ms, α=12°, FOV=45x45mm2, 15mm slice thickness) with a custom outward spiral k-space trajectory [7]. Respiratory motion was mitigated during carbon imaging by applying a 12s breath-hold. Metabolic maps were generated and voxels covering the lungs and the corresponding areas in the proton images were manually segmented into four regions (anterior, posterior, left and right: shown in figure 1) using custom-made routines in MATLAB2014b. All data were exported to R for statistical analysis.Results and Discussion
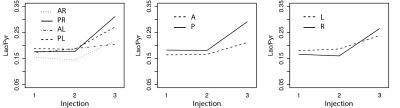
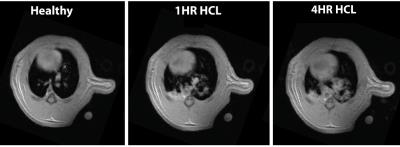
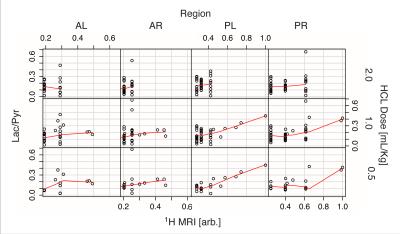
Figure 2 shows the average of the lactate-to-pyruvate ratio in the four regions of the lung as a function of injection number, demonstrating that the lactate-to-pyruvate ratio changes similarly in the left and right sides of the lung for all injections. In the posterior regions, however, the lactate-to-pyruvate ratio increases more significantly than in the anterior areas. This is consistent with the pattern of edema formation and its progression as observed with proton MRI (figure 3) and CT [1] for this injury model. The Increased lactate-to-pyruvate ratio is a result of the infiltration of neutrophils into the lungs upon injury [4-5]. It is important to note that blurring associated with cardiac motion in voxels near the heart can affect accurate quantification of the metabolites in the anterior regions of the lungs. Comparisons between the regional lactate-to-pyruvate ratio and the normalized proton signal intensity for all injured cohorts (figure 4) show a similar trend, and there appears to be a good correlation between the lactate-to-pyruvate ratio and proton signal intensity in injured-0.5 and injured-1 cohorts in the posterior regions. In the case of severe injury (injured-2) no obvious correlation is observed between the carbon and proton images. This is possibly due to dramatically increased blood flow in very severe cases of injury [6], which may lower the lactate-to-pyruvate ratio as the injury progresses [8].Conclusions
Regional analysis shows that the lactate-to-pyruvate ratio in injured lungs alters more significantly in the posterior regions than in the anterior regions. This suggests that regional lactate-to-pyruvate ratio may be a more sensitive biomarker for pulmonary inflammation, as it can show alterations that are otherwise masked by the preponderance of healthy lung in the global lactate-to-pyruvate ratio. This study shows that the development of pulse sequences capable of providing high resolution maps of pulmonary metabolism can improve the sensitivity of hyperpolarized carbon-13 as a tool to characterize lung inflammation.Acknowledgements
This work was supported by the National of Institutes of Health (NIH) R01 HL124986.References
[1] Cereda M. et al, Anesthesiology, 2016;124:121–131
[2] Mistry N. et al, MRM, 2008;59:289–297
[3] Drachman N. et al, MRM Sep 2016,
[4] Thind K. et al, Radiotherapy and Oncology 2014;110:317–322
[5] Shaghaghi et. al., NMR in biomedicine, 27(8):939-947 (2014).
[6] KENNEDY TP, et al. anesth. Analg. 1989;69:87–92.
[7] Kadlececk S, et al. The 24th Annual Meeting of ISMRM 2016.
[8] Pourfathi M. et al, American Thoracic Society, May 2016, San Francisco, CA.
Figures