3611
Optimization of redox-state assessment in rat liver using hyperpolarized [1-13C]alanine1Advanced Imaging Research Center, University of Texas Southwestern Medical Center, Dallas, TX, United States, 2Radiology, Stanford University, Stanford, CA, United States, 3Applied Science Laboratory, GE Healthcare, Menlo Park, CA, United States
Synopsis
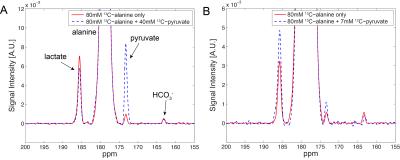
In this study, we demonstrated the strategies of increasing signal sensitivities of 13C-pyruvate and 13C-lactate generated from an injection of hyperpolarized 13C-alanine by (1) adjusting the alanine dose and (2) co-injecting unlabeled pyruvate. 120-mM alanine produced larger amount of labeled pyruvate and lactate as compared to when 80-mM or 40-mM alanine was injected. The co-injection of 7-mM unlabeled pyruvate showed up to 49% SNR increase in pyruvate and lactate peaks.
Background
The ratio of intracellular lactate and pyruvate concentrations is tightly regulated by free nicotinamide adenine dinucleotide (NAD+) and its reduced form (NADH) via lactate dehydrogenase (LDH), and has been used to estimate free cytoplasmic redox states (1). Recently, the ratio of intracellular products of [1-13C]pyruvate and [1-13C]lactate, produced from a bolus injection of hyperpolarized [1-13C]alanine, was suggested as an useful method for assessing in vivo redox levels in the liver (2). The measured pyruvate, lactate, and HCO3- signals, however, were dominated by the injected alanine, resulting in a compromised signal-to-noise ratio (SNR) of products – pyruvate, in particular. Besides the related enzyme activities and NAD+/NADH, the intracellular production of 13C-labeled pyruvate, lactate, and HCO3- is governed by two additional factors: the activity of the alanine-serine-cysteine (ASC) transporter and the intracellular pool sizes. The small 13C-pyruvate signal is likely limited by the small intrinsic pyruvate pool size in cytoplasm. Such limitations have not been investigated and, additional experiments with varying dosages of hyperpolarized 13C-alanine would be needed to optimize the SNR and reliability of the redox measurements, especially for the localized redox information obtainable with spectroscopic imaging. In this study, we tested three different dosages of [1-13C]alanine, and investigated a way to increase the metabolic pool sizes by co-injecting unlabeled pyruvate and exploiting a fast chemical exchange between pyruvate and lactate (3).Methods
Alanine samples were prepared as described in (4), mixed with 15-mM OX063 trityl, then polarized using a SPINlab™ clinical DNP polarizer (5T). Free-fed male Wistar rats (365 – 478 g, n = 5) were prepared for the study. For a dosage optimization, 40, 80, and 120-mM of [1-13C]alanine solutions (0.5, 1.0, and 1.5 mmol/kg body weight, respectively) were administered 30 s after the dissolution through the tail vein catheter at a rate of 0.25 mL/s. For augmenting the intrinsic pool sizes of pyruvate and lactate, unlabeled pyruvate (0 - control, 7, or 40-mM) was mixed with 80-mM hyperpolarized 13C-alanine immediately after dissolution: 40-mM (n = 2) and 7-mM (n = 2). Up to three alanine solutions were injected per animal with at least 1-h interval between injections. All MR measurements were performed on a clinical 3T GE Signa PET/MR scanner using a custom-built 13C RF transmit/receive surface coil (single loop, Ø = 28 mm) and dynamic free induction decay pulse sequence with a 10o hard RF pulse (pulse width = 40 μs, spectral width = 5,000 Hz, 2,048 spectral points, acquisition time = 4 min, temporal resolution = 3 s).Results and Discussion
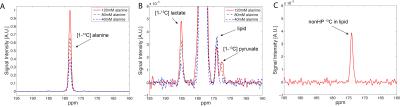
[1-13C]pyruvate/[1-13C]lactate and H13CO3-/[1-13C]lactate was measured as 0.127 and 0.057, respectively, in liver when hyperpolarized 80-mM [1-13C]alanine was injected (n = 5). When 40-mM unlabeled (12C) pyruvate was co-injected with alanine, a dramatic increase of 13C-pyruvate peak and a moderate decrease of 13C-lactate was observed (13C-pyruvate/13C-lactate = 1.240) while H13CO3-/13C-lactate was maintained at a similar level as the baseline (n = 2, Fig.1A). On the contrary, the group with an injection of 7-mM 12C-pyruvate along with hyperpolarized 80-mM [1-13C]alanine showed simultaneous increases on both 13C-lactate and 13C-pyruvate (13C-pyruvate/13C-lactate = 0.129 to 0.142), resulting in an increase of the SNRs by 49% (Fig.1B). This is likely because the 40-mM pyruvate and its fast intracellular transport via monocarboxylate transporters (MCTs) overwhelmingly augmented the pool sizes of pyruvate and lactate, disturbing the balance of intracellular metabolic system (e.g., redox-state). 7-mM is more appropriate and reasonable concentration for pyruvate, considering the small intracellular pyruvate and lactate concentrations. In the alanine dose experiment, the amount of 13C-pyruvate and 13C-lactate production was proportional to the injected 13C-alanine concentration (n = 1, Fig.2A-B). The spectra were noisier than the other data probably because of either 1) the large size of the animal (478 g), resulting in a poor coil sensitivity due to the thick lipid layer located between the liver and the coil, or 2) suboptimal RF calibration (e.g., peak B1 in the fat layer rather than the liver). Moreover, the spectra consistently detected additional 13C-signal at 174ppm even without any infusion (Fig.2C), which is probably from the natural abundance 13C signal of lipids (4). Dynamic data will be further analyzed based on a model with varying pool sizes.Conclusion
In this study, we demonstrated the possibilities of increasing the signal sensitivity of 13C-pyruvate and 13C-lactate generated from an injection of hyperpolarized 13C-alanine by optimizing the dose of alanine and co-injecting unlabeled pyruvate. Model-based kinetic analysis and further optimization will be necessary for more reliable assessment of cytoplasmic redox-state in liver.Acknowledgements
National Institute of Health (R01 CA176836, R01 EB019018, S10 OD012283, P41 EB015891) of the United States. We also thank Richard M. Lucas Foundation, GE Healthcare, and Stanford Gambhir-RSL grant.References
1. Williamson DH, Lund P, Krebs HA. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem. J. 1967;103:514–527.
2. Park JM, Liu SC, Hurd RE, Spielman DM. Noninvasive in vivo assessment of cytosolic redoxstate in rat liver using hyperpolarized [1-13C]alanine. Intl Soc Magn Reson Med 2016; 3672.
3. Hurd RE, Spielman D, Josan S, Yen Y-F, Pfefferbaum A, Mayer D. Exchange-linked dissolution agents in dissolution-DNP (13)C metabolic imaging. Magn Reson Med 2013;70:936–942. doi: 10.1002/mrm.24544.
4. Hu S, Zhu M, Yoshihara HAI, et al. In vivo measurement of normal rat intracellular pyruvate and lactate levels after injection of hyperpolarized [1-(13)C]alanine. Magn Reson Imaging [Internet] 2011;29:1035–1040. doi: 10.1016/j.mri.2011.07.001.
Figures