3610
Metabolism of Hyperpolarized Pyruvate detects Knockout of Pyruvate Dehydrogenase Kinase1Advanced Imaging Research Center, University of Texas Southwestern Medical Center, Dallas, TX, United States, 2Biochemistry, University of Texas Southwestern Medical Center, Dallas, TX, United States, 3Internal Medicine, University of Texas Southwestern Medical Center, Dallas, TX, United States, 4Chemistry, University of Texas at Dallas, Dallas, TX, United States
Synopsis
The Pyruvate Dehydrogenase Complex (PDC) plays a critical role in the regulation of carbohydrate metabolism. Pyruvate Dehydrogenase Kinase (PDK) inhibits PDC via phosphorylation making it a novel therapeutic target for metabolic diseases. The present study aimed to evaluate whether the metabolism of HP-13C pyruvate is sensitive to PDK inhibition. Our results showed that higher production of HP-bicarbonate via PDC in PDK deficient livers. 13C NMR isotopomer analysis of tissue extracts confirms higher 13C-enrichment of
Purpose
The Pyruvate Dehydrogenase Complex (PDC) converts pyruvate to acetyl-CoA and plays a critical role in regulation of carbohydrate metabolism. PDC is inhibited by Pyruvate Dehydrogenase (PDH) Kinase (PDK) via phosphorylation (1). Inhibition of PDK increases oxidative metabolism of glucose through PDC making PDK a novel therapeutic target for diabetes and other diseases. The goal of this study is to evaluate whether the production of hyperpolarized 13C-bicarbonate from [1-13C]pyruvate is sensitive to PDK inhibition. Here, we investigated pyruvate metabolism using conventional NMR and hyperpolarization methods in perfused mouse livers from PDK-deficient mice.Methods
Liver perfusion and hyperpolarized 13C NMR: Metabolism of HP [1-13C]pyruvate was investigated in fed control mice and in mice (n=3 per group) with double knockout PDK-2 and PDK-4 isoforms (DKO). Livers were excised and perfused through the hepatic portal vein using modified a Krebs-Henseleit (KH) solution containing 1.5 mM [U-13C]lactate and 0.15 mM [U-13C]pyruvate at a constant pressure of 25 cm-H2O. No other oxidizable substrates were present. The livers were perfused for 30 min. A solution of HP [1-13C]pyruvate (3 mL) pre-polarized in a HyperSense polarizer for 2 h with 15 mM OX063 and 2 mM ProHance was quickly mixed with 20 mL of Krebs-Henseleit solution and injected directly into the perfused liver through a polyethylene catheter. A series of 13C NMR spectra were acquired immediately (2 s delay time) after the injection of hyperpolarized solution using 20° pulses at 9.4 T (Varian INOVA) spectrometer equipped with a 25-mm broadband probe (Doty Scientific). After the HP NMR, the livers were perfused for additional 15 min before being freeze-clamped.
High-resolution 1H and 13C NMR spectroscopy of tissue extracts: Frozen liver tissues were extracted with 5% perchloric acid. The mixture was neutralized, freeze-dried, and reconstituted in D2O containing 0.5 mM DSS as an internal standard. NMR spectra were acquired on a 14.1 T spectrometer equipped with a 10-mm Bruker cryoprobe. Acquired data were zero filled to 32,768 points, Fourier-transformed, referenced to DSS, phase and baseline corrected using ACD/NMR Processor.
Results and Discussions
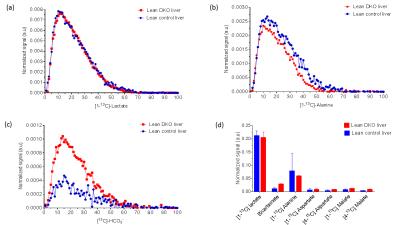
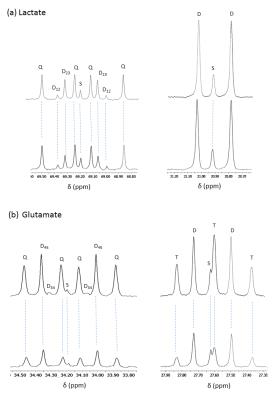
The oxygen consumption of livers ~5 mins before the injection was 1.133±0.076 and 0.902±0.495 µmol/min/g ww for control and DKO groups, respectively. Production of HP-bicarbonate was observed in both groups of livers (2). However, much higher HP-bicarbonate signal (Figs. 1-2) was observed in the DKO group. The results are most consistent with higher flux through PDC in the DKO livers. Interestingly, small signals from the carboxyl groups of malate and aspartate were more prominent in the DKO livers as well (Figures 1 and 2). The underlying metabolic state of the livers examined by 13C NMR isotopomer analysis of tissue extracts also indicates higher PDC flux in the DKO livers than the control group. The larger doublet of doublets (or quartet) in glutamate C4 and the larger doublet of doublet (or triplet) in glutamate C3 are both consistent with higher levels of enriched acetyl-CoA. The doublet due to J12 and J23 in lactate C2 can only arise under these conditions from flux through oxaloacetate (or an equivalent 4–carbon intermediate) into pyruvate via pyruvate kinase or an equivalent pathway. The activity of this pathway did not differ in the DKO livers (Figure 3).Conclusion
Increased oxidative metabolism of HP [1-13C]pyruvate through PDC was observed in PDK-deficient livers as shown by HP 13C NMR and corroborated by high-resolution 13C NMR of tissue extracts. The result suggests that the appearance of HP-bicarbonate is a sensitive biomarker for monitoring the consequences of PDK inhibition.Acknowledgements
This work supported by the NIH (5R37-HL034557 and 8P41-EB015908). Xiaodong Wen and Thomas Hever are acknowledged for their technical support in the perfusion experiments.References
(1) Zhang S, Hulver MW, McMillan RP, Cline MA, Gilbert ER. The pivotal role of pyruvate dehydrogenase kinases in metabolic flexibility. Nutrition & Metabolism. 2014; 11,10.
(2) Merritt ME, Harrison C, Sherry AD, Malloy CR, Burgess SC. Flux through hepatic pyruvate carboxylase and phosphoenolpyruvate carboxykinase detected by hyperpolarized 13C magnetic resonance. Proc Natl Acad Sci. 2011; 108, 19084-9.
Figures