3608
Fumarate to Malate Conversion in Infarcted Porcine Heart – a Pilot Study1The MR Research Centre, Aarhus University, 8200, Denmark, 2Danish Diabetes Academy, Odense, Denmark, 3Department of Cardiology, Aarhus University Hospital, 8200, Denmark
Synopsis
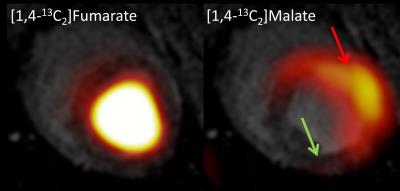
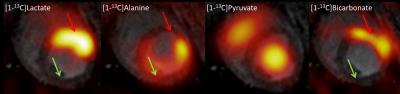
Hyperpolarized MR may be a key tool for investigation cardiac metabolism and cardiac treatment response. [1,4-13C2]Fumarate is an emerging and interesting candidate for measuring and visualizing cardiac injury after ischemia. In this study we showed an initial step for imaging cardiac cell death in a large animal model with [1,4-13C2]malate. The [1,4-13C2]malate signal correlated well with increased 13C-lactate signal and 13C-alanine absence. Overall, this shows increased metabolism in the infarcted area and ongoing necrosis.
Introduction
Acute Myocardial Infarction (AMI) is a leading cause of death globally and in the United States (US) 1. Myocardial ischemia has the highest morbidity and mortality of all disease entities in the cardio-vascular diseases1. Because of this, enormous scientific attention has long been directed at ischemia in the heart. More recently, and especially with the introduction of cardioprotection through various forms of conditioning, myocardial molecular mechanics and –metabolism have gained a key role in ischemia research2-4. Hyperpolarized MR may be a key tool for investigation in this field5,6, and numerous studies have been carried out in the last few years utilizing this technique to interrogate myocardial metabolism, showing great results, both in large animal models7,8, and, more recently, in humans9. To date, the bioprobe most frequently used has been [1-13C]Pyruvate, which, through visualization and relative quantification of its derivatives lactate, alanine and bicarbonate, can examine the breakdown of glucose in the heart and give indications of anerobic and aerobic metabolism hereof10, but more bioprobes are constantly being developed, and as such, [1,4-13C2]Fumarate has emerged as a highly interesting candidate. Fumarate examines ongoing necrosis through visualization and relative quantification of malate, which it is converted to via the fumarase enzyme – an enzyme it only has access to in the case of cell membrane rupture or severely increased cell permeability11. It could therefore be highly promising in the setting of ischemia research. In this pilot study, we employed both hyperpolarized [1-13C]Pyruvate- and [1,4-13C2]Fumarate-MR imaging in a clinically relevant, large animal model of myocardial ischemia-reperfusion injury to study the metabolic effects of AMI and to show the feasibility of the model.Methods
One healthy, female, Danish domestic pig weighing 30 kg was included in this study. The pig was anaesthetized via continuous intravenous infusion of both Propofol and Fentanyl. The pig was intubated and mechanically ventilated (with a 60% O2-air mix) using a respirator system (GE Healthcare, Denmark). Catheterizations were performed in the left femoral vein for administration of hyperpolarized 13C bioprobes and the right and left femoral arteries for the Percutaneous Intervention (PCI) -procedure and invasive blood pressure monitoring, respectively. Coronary occlusion was induced by an angioplasty balloon in the LAD distal to the second diagonal branch artery. The balloon occluded the LAD for 65 minutes. A whole-body clinical 3T GE HDx MR scanner (GE Healthcare, Milwaukee, WI, USA) was used to acquire anatomical 1H images during short breath-holds with the body coil (GE Healthcare, Milwaukee, WI, USA). A bore-insertable 13C volume resonator (clamp shell design, f0=32.12 MHz) integrated into the patient table was used for excitation (GE Healthcare, Milwaukee, WI, USA). Two flexible foam paddles with 16 receive channels was places to cover the heart (Rapid Biomedical, Rimpar, Germany). Scan parameters for the sequences were: 13C CSI, spiral cardiac triggered, 11 excitations per image, 35(pyruvate) and 10(fumarate) image repetitions, TE 1.1ms, TR 100ms, FA 15°, matrix 60x60, field of view (FOV) 150x150mm2, in-plane resolution 2.5mm, slice thickness 50mm), total scan time (60 s for pyruvate and 10 for fumarate) and CINE-LVF (TE 1ms, TR 2.9ms, FA 35°, matrix 292x292, FOV 200x200mm2, in-plane resolution 0.7mm, slice thickness 8mm. The spiral CSI acquisitions were initiated 5s after fumarate was injected and at start of injection with pyruvate, respectively. The scans were performed 2.5 hours after reperfusion. ROI analysis was done in OsiriX (Pixmeo, Switzerland) and ejection fraction (EF) was measured in Segment v2.0 (http://segment.heiberg.se). Ratios for fumarate to malate and pyruvate to its derivatives were calculated as area under the curve in ROIs in the myocardium.Results
Fumarate was located in the lumen and [1,4-13C2]malate showed dominant signal from the ischemic area in the anterior part of the myocardium. Malate ratios were 0.26 infarct vs. 0.14 remote myocardium. Lactate was highly upregulated in the ischemic area and alanine was diminished in that area. There was little bicarbonate signal from the ischemic area, none in the remote myocardium. Lactate ratios were 0.05 infarct vs. 0.03 remote myocardium. Alanine ratios were 0.06 infarct vs. 0.09 remote myocardium. Bicarbonate ratios were 0.04 infarct vs. 0.02 remote myocardium. EF was 45% after ischemia. Normal EF for pigs is 60%.Conclusion
In this study we showed an initial step for imaging cell death in a large animal cardiac I/R model with [1,4-13C2]malate. The [1,4-13C2]malate signal correlated well with increased 13C-lactate signal and 13C-alanine absence. Overall, this shows increased metabolism in the infarcted area and ongoing necrosis. Future investigations for this method and model will focus on optimal timing after reperfusion as well as improved imaging of [1,4-13C2]malate.Acknowledgements
Funded by The Danish Diabetes Academy supported by the Novo Nordisk Foundation.References
1. Writing Group Members, Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, et al. Executive Summary: Heart Disease and Stroke Statistics--2016 Update: A Report From the American Heart Association. Circulation. American Heart Association, Inc; 2016 Jan 26;133(4):447–54.
2. Lopaschuk GD, Ussher JR, Folmes CDL, et al. Myocardial Fatty Acid Metabolism in Health and Disease. Physiological Reviews. 2010 Jan 19;90(1):207–58.
3. Stanley WC, Lopaschuk GD, Hall JL, McCormack JG. Regulation of myocardial carbohydrate metabolism under normal and ischaemic conditions. Potential for pharmacological interventions. Cardiovascular Research. 1997 Feb;33(2):243–57.
4. Lopaschuk GD, Stanley WC. Glucose metabolism in the ischemic heart. Circulation. 1997 Jan 21;95(2):313–5.
4. Taegtmeyer H, Young ME, Lopaschuk GD, et al. Assessing Cardiac Metabolism: A Scientific Statement From the American Heart Association. Circulation Research. 2016 May 13;118(10):1659–701.
5. Rider OJ, Tyler DJ. Clinical Implications of Cardiac Hyperpolarized Magnetic Resonance Imaging. Journal of Cardiovascular Magnetic Resonance. Journal of Cardiovascular Magnetic Resonance; 2013 Oct 8;15(1):1–1.
6. Tyler DJ, Neubauer S. Science to Practice: Hyperpolarized Metabolic MR Imaging--The Light at the End of the Tunnel for Clinical (13)C MR Spectroscopy? Radiology. 2016 Mar;278(3):639–41.
7. Schroeder MA, Lau AZ, Chen AP, et al. Hyperpolarized (13)C magnetic resonance reveals early- and late-onset changes to in vivo pyruvate metabolism in the failing heart. European Journal of Heart Failure. 2013 Feb;15(2):130–40.
8. Flori A, Liserani M, Frijia F, et al. Real-time cardiac metabolism assessed with hyperpolarized [1-(13) C]acetate in a large-animal model. Contrast Media Mol Imaging. 2015 May;10(3):194–202.
9. Cunningham CH, Lau JY, Chen AP, et al. Hyperpolarized 13C Metabolic MRI of the Human Heart: Initial Experience. Circulation Research. American Heart Association, Inc; 2016 Sep 15;:CIRCRESAHA.116.309769.
10. Schroeder MA, Clarke K, Neubauer S, Tyler DJ. Hyperpolarized magnetic resonance: a novel technique for the in vivo assessment of cardiovascular disease. Circulation. 2011 Oct 4;124(14):1580–94.
11. Gallagher FA, Kettunen MI, Hu D-E, et al. Production of hyperpolarized [1,4-13C2]malate from [1,4-13C2]fumarate is a marker of cell necrosis and treatment response in tumors. Proc Natl Acad Sci USA. 2009 Nov 24;106(47):19801–6.
Figures