3606
Acute afterload-imposed change in porcine cardiac metabolism imaged by hyperpolarized [1-13C]Pyruvate1Department of Cardiology - Research, Aarhus University Hospital, Aarhus N, Denmark, 2MR Research Centre, Aarhus University, Aarhus N, Denmark
Synopsis
Deranged metabolism is now considered a key causal factor in heart failure and has therefore gained considerable scientific interest. The novel technique hyperpolarized MR has emerged as a leading methodological candidate to study these derangements. We employed a clinically relevant, large animal model of angiotensin-II-mediated acute hypertension to study cardiac metabolism in the setting of elevated afterload using hyperpolarized [1-13C]Pyruvate MR. The method was able to detect acute increases in both anaerobic and aerobic cardiac metabolism, which, in the future could mean a useful way of monitoring a possible treatment response to afterload reduction by using hyperpolarized MR.
Purpose
Deranged metabolism is now being viewed as a key causal factor in heart failure1-3. It has therefore garnered much interest in recent years, owing to the belief that a better understanding of metabolism in heart failure, could potentially better patients’ prognosis1-3, which, despite recent advancements, remains poor4,5. However, results from animal studies and clinical trials have displayed immense heterogeneity1-3. This can, in part, be explained by methodological challenges in studying the transient states of metabolism. These challenges can possibly be overcome by the novel technique hyperpolarized MR6-9. Afterload changes and the renin-angiotensin-aldosterone-system (RAAS) play a major part in the pathology of heart failure, and, accordingly, afterload reduction and RAAS-inhibition is a cornerstone in current heart failure treatment10. We employed a clinically relevant, large animal model of acute angiotensin-II-mediated hypertension to study the dynamic effects of afterload changes on cardiac metabolism.Methods
5 pigs weighing 30 kg. were fed on the morning of the experiments, anaesthetized with propofol and fentanyl and mechanically ventilated. Details of the study workflow can be seen in Figure 1. All experiments were performed on a clinical 3T GE HDx MR scanner (GE Healthcare, Chicago, Illinois, USA). Proton-images were acquired using an 8-channel cardiac array receiver coil (GE Healthcare, Chicago, Illinois, USA) and 13C-data was acquired with a 13C Helmholtz loop coil of 20cm in diameter (PulseTeq Limited, Surrey, UK). The scan protocol was: proton scout sequence, CINE left ventricular function, Bloch-Siegert sequence, and finally a spiral CSI sequence 30 seconds after injection of pyruvate. All scans were ECG-triggered in the diastolic phase and performed during breath-hold. Vital parameters were recorded and blood samples were obtained throughout the study. DICOM images were analyzed in OsiriX MD version 7.0.1 (Pixmeo SARL, Bernex, Switzerland). ROIs were drawn to outline the myocardium, excluding signal from the lumen. Results were calculated as summed signal from single metabolites – lactate, alanine and bicarbonate, respectively – in the myocardium over the course of the entire scan sequence. This was then divided by (normalized to) firstly; summed pyruvate- and, secondly; summed alanine-signal, as we anticipated the hemodynamic changes to increase variance in the pyruvate signal and wanted to exploit alanine being a marker for intracellular pyruvate11-14. Statistics performed were t-tests and one-way ANOVA with post hoc Holm-Sidak's multiple comparisons test.
Results
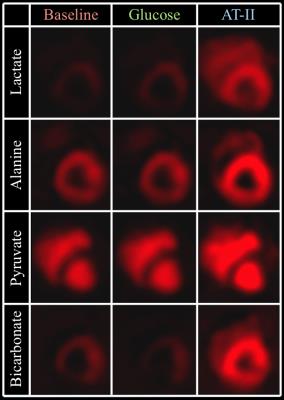
Image quality was consistenly good, see Figure 3. Lactate/alanine- and bicarbonate/alanine-ratios were both significantly increased at final time point, while no differences were observed when normalizing to pyruvate, see Figure 4. No effect of sugar bolus was seen with hyperpolarized [1-13C]Pyruvate MR in spite of both blood sugar-, serum pyruvate- and insulin levels increasing throughout the study (ANOVA p=0.0283, p=0.0004 and p=0.0103), see Figure 2B. Free Fatty Acid (FFA)-levels remained unchanged (p=0.1280). End-tidal CO2 (p=0.0025) and arterial lactate-levels (p=0.0052) increased during the study, while arterial pH showed a tendency to decrease that did not reach statistical significance (p=0.0615). Results are presented as mean±SD. Blood pressures increased significantly, with MAP increasing ≈180% from 62.6±6.58mmHg at time point 150 to 112±9.67mmHg at time point 180 (p=0,0076). Ejection Fraction (EF) had decreased 7.2±4.7 percentage points at time point 180 (p=0.0270), while heart rate increased throughout the study (ANOVA p=0.0088), see Figure 2A.Discussion
The study demonstrates the ability of hyperpolarized [1-13C]Pyruvate MR to detect rapid changes in cardiac metabolism imposed by acutely elevated afterload. Interestingly, these findings were not supported by our data normalized to pyruvate, but were only apparent when normalizing to alanine. Alanine has been proposed as a marker for intracellular pyruvate11-14., and as such, normalizing to alanine may provide greater sensitivity to myocardial rates of enzymatic conversion, since any increased variance stemming from the pyruvate in the extracellular matrix (ECM) is eliminated by use of this method. This is perhaps especially true in the setting of rapidly changing hemodynamics, where perfusion pressures, and thus pyruvate in the ECM, will likely vary greatly.
Conclusion
In this study, we demonstrate the ability of hyperpolarized [1-13C]Pyruvate MR to detect an acute change in cardiac metabolism imposed by of increased afterload. This study was performed in a large animal, clinically relevant model of acute hypertension and we demonstrated superior sensitivity of our metabolite data upon normalization to alanine. This is relevant for designing future studies with altered hemodynamics and entails the possibility of evaluating response to afterload-lowering treatment directly via cardiac metabolism using hyperpolarized [1-13C]Pyruvate MR. Our findings did not support cardiac glucose metabolism changing as a response to the oral sugar bolus. This is relevant for designing both future chronic animal studies and human trials with hyperpolarized [1-13C]Pyruvate MR.Acknowledgements
The authors wish to thank Henrik Vestergaard Nielsen for invaluable technical assistance in preparing the pyruvate samples and operating the SpinLab. Likewise, Per Mose Nielsen and Casper Carlsen Elkjær are to be thanked for assisting with blood sample analysis.
This study was funded by The Danish Heart Foundation, Aarhus University, Karen Elise Jensen’s Foundation and Director Kurt Bønnelycke and Wife’s Foundation
References
1. Neubauer S. The failing heart--an engine out of fuel. The New England journal of medicine. 2007;356(11):1140–1151.
2. Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiological Reviews. 2005;85(3):1093–1129.
3. Lopaschuk GD, Ussher JR, Folmes CDL, Jaswal JS, Stanley WC. Myocardial Fatty Acid Metabolism in Health and Disease. Physiological Reviews. 2010;90(1):207–258.
4. Writing Group Members, Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després J-P, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, et al. Executive Summary: Heart Disease and Stroke Statistics--2016 Update: A Report From the American Heart Association. Circulation. 2016;133(4):447–454.
5. McMurray JJV, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Køber L, Lip GYH, Maggioni AP, Parkhomenko A, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. European heart journal. 2012;33(14):1787–1847.
6. Ardenkjaer-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, Servin R, Thaning M, Golman K. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(18):10158–10163.
7. Tyler DJ. Cardiovascular Applications of Hyperpolarized MRI. Current cardiovascular imaging reports. 2011;4(2):108–115.
8. Rider OJ, Tyler DJ. Clinical Implications of Cardiac Hyperpolarized Magnetic Resonance Imaging. Journal of Cardiovascular Magnetic Resonance. 2013;15(1):1–1.
9. Tyler DJ, Neubauer S. Science to Practice: Hyperpolarized Metabolic MR Imaging--The Light at the End of the Tunnel for Clinical (13)C MR Spectroscopy? Radiology. 2016;278(3):639–641.
10. Hartupee J, Mann DL. Neurohormonal activation in heart failure with reduced ejection fraction. Nature reviews. Cardiology. 2016.
11. Lewandowski ED. Metabolic heterogeneity of carbon substrate utilization in mammalian heart: NMR determinations of mitochondrial versus cytosolic compartmentation. Biochemistry. 1992;31(37):8916–8923.
12. Peuhkurinen KJ, Hiltunen JK, Hassinen IE. Metabolic compartmentation of pyruvate in the isolated perfused rat heart. The Biochemical journal. 1983;210(1):193–198.
13. Peuhkurinen KJ, Nuutinen EM, Pietiläinen EP, Hiltunen JK, Hassinen IE. Role of pyruvate carboxylation in the energy-linked regulation of pool sizes of tricarboxylic acid-cycle intermediates in the myocardium. The Biochemical journal. 1982;208(3):577–581.
14. Atherton HJ, Schroeder MA, Dodd MS, Heather LC, Carter EE, Cochlin LE, Nagel S, Sibson NR, Radda GK, Clarke K, Tyler DJ. Validation of the in vivo assessment of pyruvate dehydrogenase activity using hyperpolarised 13C MRS. NMR in Biomedicine. 2011;24(2):201–208.
Figures
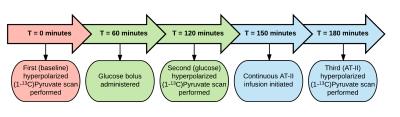
Flowchart depicting the experimental protocol.
All pigs were scanned with hyperpolarized [1-13C]Pyruvate cardiac MR at baseline, given a sugar bolus in the stomach and scanned again 1 hour later. They were then continuously infused with angiotensin II intravenously to elevate afterload for 30 minutes and a final scan was performed.
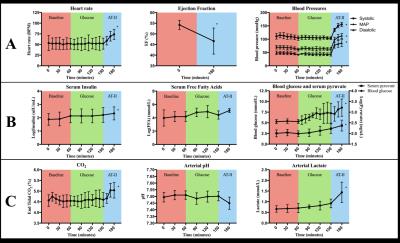
An overview of vital parameters and blood samples. * denotes statistical significance. See Results-section for details.
A: Heart rate, ejection fraction and blood pressures. EF=ejection fraction, MAP=mean arterial pressure.
B: Serum insulin, free fatty acids and pyruvate as well as blood glucose. The three former have been log-transformed to achieve normality. FFA=free fatty acids.
C: End-tidal CO2, arterial pH and arterial lactate.
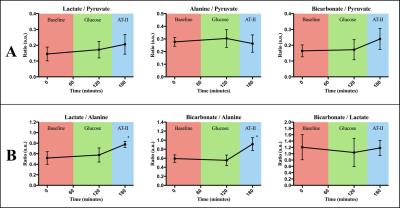
Results in ratios. (a.u.) denotes arbitrary units. * denotes statistically significant difference from earlier time points.
Ratios are seen to be constant when normalizing to pyruvate (A) but increases at final time point when normalizing to alanine (B).