3605
Metabolic changes in the heart precede functional changes in a rat model of doxorubicin-induced cardiotoxicity1Physiology Anatomy and Genetics, University of Oxford, Oxford, United Kingdom
Synopsis
Chemotherapeutic agents such as doxorubicin can cause serious adverse effects on the heart, leading to decreased left ventricular function and heart failure. The biochemical mechanisms for this are not fully understood, however, increased oxidative stress in cardiomyocytes as well as bioenergetic changes to the heart have been suggested as primary triggers for the functional decline. Here we show that hyperpolarized 13C magnetic resonance spectroscopy and CINE magnetic resonance imaging of the heart can detect metabolic as well as functional changes in a clinically relevant rat model of doxorubicin-induced cardiotoxicity, and that metabolic changes may precede functional abnormalities.
Purpose
Common anthracycline chemotherapeutics such as doxorubicin (DOX) can cause serious cardiotoxic side effects, which can lead to decreased left ventricular function and heart failure. The functional changes are thought to be caused by a combination of biochemical changes to the cardiomyocytes, including damage due to oxidative stress, and changes in cardiac bioenergetics1. Changes in substrate utilization and metabolic fluxes lead to inadequate energy reserves in the heart, which can cause cardiac remodeling and functional deficits2. Dissolution dynamic nuclear polarization and magnetic resonance spectroscopy of injected hyperpolarized substrates has been successfully applied to the preclinical study of cardiac pathologies3,4. We have previously shown early changes in cardiac metabolism and function in a rat model of DOX-induced cardiotoxicity using high-dose i.p. injections5. In this study we investigated whether serial low-dose i.v. injections of doxorubicin, that closely mimic clinical chemotherapy protocols, also lead to metabolic changes in the rat heart and if these metabolic changes precede functional impairment.Methods
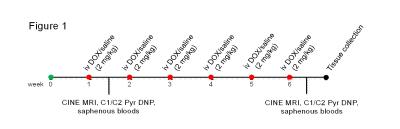
Weight-matched male Wistar rats were treated weekly for six weeks with i.v. injection of either saline (n=8) or 2 mg kg-1 DOX (Apollo Scientific) dissolved in saline (n=8) (Figure 1). Rats were weighed daily for two weeks and then twice weekly for the remaining time. At one and six weeks after the first dose, functional CINE MR imaging and hyperpolarized [1-13C]- and [2-13C]pyruvate magnetic resonance spectroscopy were performed on a 7T spectrometer (Varian). CINE images were acquired as described previously6. [1-13C]- and [2-13C]pyruvate were hyperpolarized as described previously7 and 1 mL of 80 mM pyruvate was injected into the tail vein over 10 s. 13C MR spectra were acquired every second for 60 s using a 72-mm dual-tuned birdcage volume transmit 1H/13C coil and a 13C two-channel surface receive coil (Rapid Biomedical; 10º hard pulse; 10 kHz bandwidth). Multicoil spectra were added in phase, and the first 30 s of spectra from appearance of the pyruvate peak were summed and quantified with AMARES/jMRUI for ratiometric analysis8. Blood was collected from the saphenous veins of anaesthetized rats at the end of week one and six. Rat cardiac troponin I (cTNI) levels were measured in the plasma using an ELISA kit (Life Diagnostics). One week after the last dose rats were sacrificed and their intra-abdominal fat pads weighed and tibial length measured. Experiments were performed following ethical approval.Results
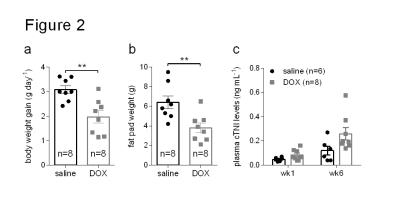
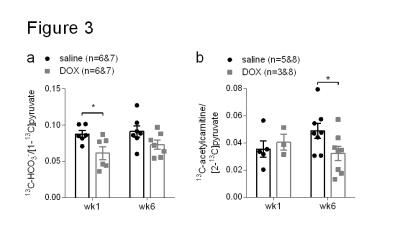
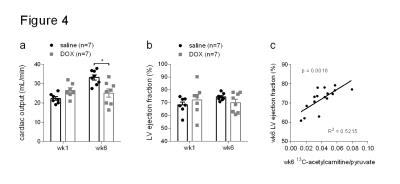
Rats treated for six weeks with weekly i.v. injections of doxorubicin showed decreased weight gain and less intra-abdominal fat than control rats treated with saline (Figure 2a and b). At six weeks after the first dose, DOX-treated rats also had higher levels of plasma cardiac troponin I (cTNI) (Figure 2c), although this was not statistically significant (p=0.06). After the first dose DOX-treated rats showed a decreased 13C-HCO3-/[1-13C]pyruvate ratio in the heart (Figure 3a). After six weeks this ratio remained low, although the reduction was not statistically significant (p=0.09). After six weeks, hearts from DOX-treated rats also showed a decreased 13C-acetylcarnitine/[2-13C]pyruvate ratio (Figure 3b). The metabolic changes at week six were accompanied by functional changes, as reflected by a decreased cardiac output (Figure 4a). Cardiac left ventricular ejection fraction (EF) was not significantly altered (Figure 4b), however, EF correlated with the reduced ratio of 13C-acetylcarnitine/[2-13C]pyruvate at week six (Figure 4c).Discussion
DOX-treatment led to decreased weight gain and reduced fat reserves, which was shown to be due to reduced adipogenesis and PPARγ-mediated uptake of glucose and fatty acids into adipose tissue9. DOX preferentially binds to cardiolipin, a phospholipid in the inner mitochondrial membrane, and this leads to impaired mitochondrial energy metabolism due to oxidative damage of nearby membrane proteins1. Pyruvate dehydrogenase (PDH), the linker enzyme between glycolysis and the tricarboxylic acid (TCA) cycle, is located in the inner mitochondrial membrane and its functional impairment might explain the decreased labeling of 13C-HCO3-, the product of PDH, which we saw also in our high-dose i.p. model5. We also saw decreased levels of 13C-acetylcarnitine after six weeks of DOX-treatment in the heart and DOX has been shown to decrease levels of L-carnitine in cardiomyocytes as well as decreasing fatty acid oxidation10. DOX treatment also led to reduced cardiac output, which has been shown previously in rats treated weekly for six weeks with 2.5 mg kg-1 DOX11.Conclusions and future work
Metabolic changes in this clinically relevant rat model of DOX-induced cardiotoxicity seem to precede functional changes, and these metabolic changes can be assessed with hyperpolarized [1-13C]pyruvate and [2-13C]pyruvate. Future work will comprise trying to prevent early changes in metabolism with drugs such as dichloroacetate to test whether this can prevent late functional deficits.Acknowledgements
This work was supported by the British Heart Foundation.References
1. Rochette L, Guenancia C, Gudjoncik A, et al. Anthracyclines/trastuzumab: new aspects of cardiotoxicity and molecular mechanisms. Trends Pharmacol Sci 2015;36:326-48.
2. Taegtmeyer H, Young ME, Lopaschuk GD, et al. Assessing Cardiac Metabolism: A Scientific Statement From the American Heart Association. Circ Res 2016;118:1659-701.
3. Schroeder MA, Atherton HJ, Ball DR, et al. Real-time assessment of Krebs cycle metabolism using hyperpolarized 13C magnetic resonance spectroscopy. FASEB 2009;23:2529-38.
4. Schroeder MA, Cochlin LE, Heather LC, Clarke K, Radda GK, Tyler DJ. In vivo assessment of pyruvate dehydrogenase flux in the heart using hyperpolarized carbon-13 magnetic resonance. PNAS 2008;105:12051-6.
5. Dodd MS, Ball V, Tyler DJ. Metabolic alterations in chemotherapy-induced cardiotoxicity revealed by in vivo hyperpolarized 13C-MRS. Proc. Int. Soc. Mag. Reson. Med. 24 (2016);2536
6. Dodd MS, Ball DR, Schroeder MA, et al. In vivo alterations in cardiac metabolism and function in the spontaneously hypertensive rat heart. Cardiovascular research 2012;95:69-76.
7. Dodd MS, Atherton HJ, Carr CA, et al. Impaired in vivo mitochondrial Krebs cycle activity after myocardial infarction assessed using hyperpolarized magnetic resonance spectroscopy. Circulation Cardiovascular imaging 2014;7:895-904.
8. Vanhamme L, van den Boogaart A, Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson 1997;129:35-43.
9. Arunachalam S, Kim SY, Kim MS, et al. Adriamycin inhibits adipogenesis through the modulation of PPARgamma and restoration of adriamycin-mediated inhibition of adipogenesis by PPARgamma over-expression. Toxicol Mech Methods 2012;22:540-6.
10. Abdel-aleem S, el-Merzabani MM, Sayed-Ahmed M, Taylor DA, Lowe JE. Acute and chronic effects of adriamycin on fatty acid oxidation in isolated cardiac myocytes. J Mol Cell Cardiol 1997;29:789-97.
11. Xiang P, Deng HY, Li K, et al. Dexrazoxane protects against doxorubicin-induced cardiomyopathy: upregulation of Akt and Erk phosphorylation in a rat model. Cancer Chemother Pharmacol 2009;63:343-9.
Figures