3604
Imaging branched-chain amino acid metabolism in glioma using hyperpolarized [1-13C] alpha-ketoisocaproate1Advanced Imaging Research Center, University of Texas Southwestern Medical Center, Dallas, TX, United States, 2Organ Transplantation Center, Sun Yat-sen University, Guangzhou, People's Republic of China, 3Chemistry, University of Texas Dallas, Richardson, TX, United States
Synopsis
Upregulated branched-chain amino transaminase 1 (BCAT1) expression is a common metabolic feature of most primary cancers with wild-type isocitrate dehydrogenase (IDH), including glioblastoma. In this study, 13C-labeled a-ketoisocaproate (KIC) metabolism was investigated in a brain tumor-bearing rat to assess BCAT1 and branched-chain α-ketoacid dehydrogenase (BCKDH) activities in the tumor. Following an intravenous bolus injection of hyperpolarized [1-13C]KIC, both [1-13C]leucine and 13C-bicarbonate were observed in the brain. We observed less [1-13C]leucine but greater bicarbonate production in the tumor compared to normal, healthy brain tissue, suggesting downregulated chemical exchange of [1-13C]KIC with leucine catalyzed by BCAT1 and upregulated BCKDH activity, respectively.
Background
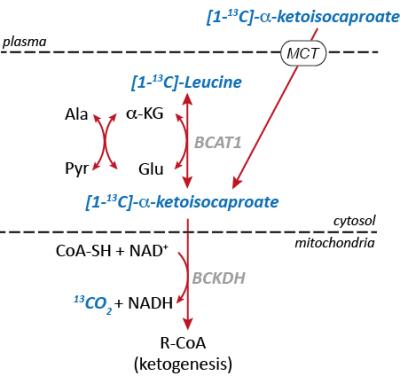
13C MRSI in combination with dynamic nuclear polarization (DNP) provides opportunities to noninvasively monitor biochemical information in metabolic pathways of interest using hyperpolarized 13C-enriched substrates. Altered utilization of branched-chain amino acids (BCAA) has been studied in multiple cancer models so detection of BCAA activity may serve as a potential biomarker of tumor metabolism [1]. Moreover, increased activities of branched-chain amino transaminase 1 (BCAT1) and branched-chain α-ketoacid dehydrogenase (BCKDH) have been reported in glioblastomas with wild-type isocitrate dehydrogenase (IDHwt), thereby enabling cell proliferation by catabolism of BCAAs [2]. Recently, hyperpolarized [1-13C] α-ketoisocaproate (KIC) and its conversion to [1-13C]leucine was correlated with BCAT activity in lymphoma [3] and in normal rat brain [4]. In particular, in the brain, the BCAAs (valine, leucine, and isoleucine) provide nitrogen for the synthesis of glutamate via BCAT1 and the resulting branched-chain a-ketoacids (BCKA) can be further catabolized to 13CO2/bicarbonate (H13CO3-) via BCKDH (Fig. 1). In this study, we examined the potential of hyperpolarized [1-13C] KIC as a metabolic probe of altered BCAT1 and BCKDH-catalyzed reactions in glioma.Methods
Approximately 104 F98 rat glioma cells were implanted into the right striatum of male Fischer rats 15-18 days prior to the 13C imaging (n=3, body weight = 216-222g). A 2D spiral chemical shift imaging pulse sequence with a variable RF scheme [5] was used to acquire single time-point metabolite maps of rat brain (four spatial interleaves, spectral bandwidth = 210.8 Hz, #echoes = 64, field of view = 5×5 cm2, nominal spatial resolution = 3.1×3.1 mm2, slice thickness = 8 mm, acquisition time = 2 s) following an intravenous injection of 84-mM (2.5mL) hyperpolarized [1-13C]KIC (injection-to-scan time = 30 s, polarized in a SPINlab). B0 field inhomogeneity over the brain was minimized adjusting linear shim currents with 1H PRESS sequence. A custom-made surface coil (diameter = 28 mm) was used in a 3T GE 750w clinical MR scanner. Each metabolite was separately reconstructed using spectral tomosynthesis in MATLAB [6].Results and Discussion
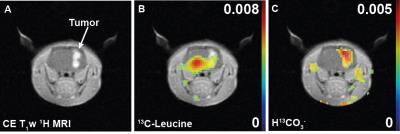
Upon intravenous bolus injection of [1-13C]KIC (δ13C=172.6ppm) into F98 glioma rats, 13C-leucine (176.8ppm) and 13C-bicarbonate (163.1ppm) were detectable in the rat brain (Fig. 2) with efficient cellular uptake of KIC via MCTs [7]. The 13C signal of the transamination product 13C-leucine was lower in the tumor compared to the contralateral normal-appearing brain (leucine/tC = 0.0053 in tumor vs. 0.0081 in normal brain, Fig.2-3), possibly due to predominant net conversion of existing cerebral leucine to KIC and associated depletion of leucine pool or rapid conversion of the injected [1-13C]KIC to the corresponding acyl-CoA and HCO3- via BCKDH. Interestingly, more bicarbonate, the decarboxylation product of [1-13C]KIC, was observed in the tumor (HCO3-/tC = 0.0050) compared to the normal brain (0.0027) presumably due to higher BCKDH activity in glioma. This suggests that BCKA catabolism by BCKDH in tumor was stimulated. The amount of leucine produced in normal brain, however, was less than that reported previously in Wistar rat brains [4],[8]. In this study, we demonstrated the feasibility of using hyperpolarized [1-13C]KIC to assess both BCAT and BCKDH activities in glioma. In future studies, we plan to investigate the metabolic kinetics and evaluate tissue distribution of BCAT/BCKDH and BCAA catabolism in normal brain and glioma using 13C isotope tracer experiments. Estimating BCAT/BCKDH enzyme kinetics in vivo could provide novel insights and strategies for diagnosis and prognosis of glioma.Conclusion
We have shown that the altered in vivo BCAA metabolism in glioma by observing 13C-leucine and 13C-bicarbonate conversion from hyperpolarized [1-13C]KIC via BCAT and BCKDH, respectively.Acknowledgements
Cancer Prevention & Research Institute of Texas (RP140021-P2) and National Institutes of Health of the United States (P41 EB015908, R37 HL034557, P01 DK058398-11A1).References
1. Baracos, V.E. and M.L. Mackenzie, Investigations of branched-chain amino acids and their metabolites in animal models of cancer. J Nutr, 2006. 136(1 Suppl): p. 237s-42s.
2. Tonjes, M., et al., BCAT1 promotes cell proliferation through amino acid catabolism in gliomas carrying wild-type IDH1. Nat Med, 2013. 19(7): p. 901-908.
3. Karlsson, M., et al., Imaging of branched chain amino acid metabolism in tumors with hyperpolarized 13C ketoisocaproate. Int J Cancer, 2010. 127(3): p. 729-36.
4. Butt, S.A., et al., Imaging cerebral 2-ketoisocaproate metabolism with hyperpolarized (13)C magnetic resonance spectroscopic imaging. J Cereb Blood Flow Metab, 2012. 32(8): p. 1508-14.
5. Park, J.M., et al., Metabolite kinetics in C6 rat glioma model using magnetic resonance spectroscopic imaging of hyperpolarized [1-(13)C]pyruvate. Magn Reson Med, 2012. 68(6): p. 1886-93.
6. Mayer, D., et al., Application of subsecond spiral chemical shift imaging to real-time multislice metabolic imaging of the rat in vivo after injection of hyperpolarized 13C1-pyruvate. Magn Reson Med, 2009. 62(3): p. 557-64.
7. Broer, S., et al., Characterization of the high-affinity monocarboxylate transporter MCT2 in Xenopus laevis oocytes. Biochem J, 1999. 341 ( Pt 3): p. 529-35.
8. Josan, S., et al., Effects of isoflurane anesthesia on hyperpolarized (13)C metabolic measurements in rat brain. Magn Reson Med, 2013. 70(4): p. 1117-24.
Figures