3601
Characterizing the Tumor Microenvironment1JHU ICMIC Program, Division of Cancer Imaging Research, The Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, MD, United States
Synopsis
Total choline in tumors is associated with increased aggressiveness. Since the distribution of total choline in tumors is usually heterogenous, here we have examined the relationship between high and low total choline, obtained with 1H MRSI, with hypoxia and necrosis in a human breast cancer xenograft model engineered to express red fluorescence protein under hypoxic conditions. We found that the highest total choline regions were associated with hypoxia. We also observed that overall total choline in tumors inversely correlated with the necrotic fraction, suggesting that reduced total choline may reflect increased cell death in tumors.
Introduction
Solid tumors frequently exhibit intratumoral hypoxia that results in an aggressive phenotype due to dysregulated gene expression and metabolic changes such as altered glycolysis [1,2], and increase in choline containing metabolites [3-6]. These metabolic alterations are mediated by activation of hypoxia inducible factors 1 and 2 (HIFs) [1] that can protect cancer cells from radiation and chemotherapy [7-8] and create stem like cancer cells [9]. Here, using tumors derived from genetically engineered MDA-MB-231 breast cancer cells that expressed red fluorescence protein (RFP) under the control of the hypoxia response element (231-HRE-RFP) in a xenograft model, we investigated the association between high and low total choline tumor areas and hypoxia. We are also characterizing the fraction of high CD44 expressing cells, a marker of stem like breast cancer cells, in high and low total choline regions.Methods
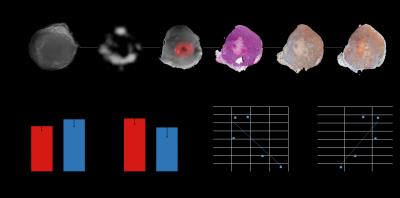
Approximately 2x106 231-HRE-RFP cells were injected in the mammary fat pad of female severely compromised immunodeficient (SCID) mice. Once the tumors were 500 mm3, MR imaging was performed with a Bruker horizontal bore 9.4T animal MR scanner using a home-built RF resonator, using a fast spin echo sequence, and 1H MR spectroscopic imaging (MRSI) of the total choline distribution was performed with a spin echo CSI sequence with water suppression (TE/TR = 80/1000ms), to obtain total choline maps from a 4 mm thick slice with an in-plane resolution of 1 mm x 1 mm. Optical imaging (Nikon fluorescence microscope) was performed to detect hypoxia in fresh tissue slices, followed by immunohistochemistry (IHC) staining for HIF-1α, and CD44 expression in 5µm thick adjacent sections from paraffin embedded 231-HRE-RFP tumors. IHC sections were scanned at high resolution with an Image Scope scanner. The optical images and IHC images were co-registered to the corresponding MR data using an in-house program in MATLAB. Co-localization analysis was performed, by drawing high and low total choline intensity masks to quantify amount of strong HIF staining in these regions of interest (ROI). Quantification for HIF-1α nuclear staining was performed by drawing these ROIs on scanned images using manufacturer supplied macro (Aperio Technologies Inc.). Correlation between total choline and necrosis and total choline and normoxic viable cells was also determined for each tumor.Results and Discussion
RFP expression was observed in our optical images confirming tumor hypoxia (Figure 1A). These RFP hypoxic tumor regions also contained some necrosis as evident in the H&E section. Immunostaining of adjacent tumor sections for HIF-1α and CD44 supported an association between HIF-1α and CD44 expression (Figure1A). To further investigate if high total choline is associated with high HIF-1α expression, we used co-localization analysis to compare HIF-1α expression in these regions. We found that that high total choline regions had higher number of strong positive HIF-1α stained cells compared to the low choline regions when the necrotic regions were eliminated (compare Figures 1B and C). A negative correlation was observed with the total choline intensity per unit area and the necrotic fraction (Figure 1D), while a positive correlation was obsereved between the total choline intensity per unit and the fraction of normoxic viable tumor regions (Figure 1E). Our data suggest that the heterogenieity of total choline in tumors is driven, in part, by hypoxia and necrosis. Total choline averaged over the entire tumor showed a significant correlation with the necrotic fraction indicating that tumors with low total choline have a high necrotic fraction.Acknowledgements
This work was supported by NIH R01CA136576 and P50 CA103175. We thank Mr. Gary Cromwell for valuable technical assistance.References
[1] Lu J, GreenwoodTR, Artemov A et al.,: Localized Hypoxia Results in Spatially Heterogeneous Metabolic Signatures in Breast Tumor Models. Neoplasia. 2012 Aug; 14(8): 732–741. [2] Brahimi-Horn MC, Chiche J, Pouyssegur J: Hypoxia signalling controls metabolic demand. Curr Opin Cell Biol 2007, 19(2):223-229. [3] Shah T, Krishnamachary B, Wildes F et al.,: HIF isoforms have divergent effects on invasion, metastasis, metabolism and formation of lipid droplets. Oncotarget. 2015 Sep 29;6(29):28104-19. [4] Glunde K, Shah T, Winnard PT Jr, et al.,: Hypoxia regulates choline kinase expression through hypoxia-inducible factor-1 alpha signaling in a human prostate cancer model.Cancer Res. 2008 Jan 1;68(1):172-80. [5] Glunde K1, Bhujwalla ZM, Ronen SM: Choline metabolism in malignant transformation. Nat Rev Cancer. 2011 Nov 17;11(12):835-48. [6] Franca P. Tumour phospholipid metabolism. NMR Biomed. 1999;12(7):413-39. [7] Gray LH, Conger AD, Ebert M, et al. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol. 1953;26(312):638-48 [8] Cosse JP, Michiels C. Tumour Hypoxia Affects the Responsiveness of Cancer Cells to Chemotherapy and Promotes Cancer Progression. Anticancer Agents Med Chem. 2008;8(7):790-7 [9] Krishnamachary B, Penet MF, Nimmagadda S, et al. Hypoxia Regulates CD44 and Its Variant Isoforms through HIF-1α in Triple Negative Breast Cancer. Plos One. 2012; 7(8): e44078.
Figures