3597
Precise longitudinal MRI tracking of systemically infused dual labelled mesenchymal stem cells and their regenerative potential in traumatic brain injury mice1NMR Research Centre, Institute of Nuclear Medicine and Allied Sciences, Delhi, India, Delhi, India, 2Division of Stem Cell and Gene Therapy Research, Institute of Nuclear Medicine and Allied Sciences, Delhi, India
Synopsis
Stem cells transplantation has emerged as a promising alternative therapeutic due to its potency at injury site. Thus, tracking of stem cells is very essential. Here, we have described a serial in vivo tracking of implanted stem cells through 7T MRI and its differentiation potential into neuronal precursors. T2*-weighted images and relaxation study demonstrated a significant signal loss and effective decrease in T2* relaxation time on day-3 at injury site. Expression of neuronal markers like GFAP, MAP2 and NeuN were observed in transplanted MSCs. The proposed procedure could be extrapolated for stem cell tracking and therapies in various neurodegenerative diseases.
Introduction
Traumatic brain injury (TBI) is a more complex biological process and there is no clinical treatment available till now1. Mesenchymal stem cells (MSCs) are multipotent in nature and have the potential to differentiate into various mesodermal tissue lineages2. To improve functional recovery, a large number of MSCs should reach and survive at injury site, and differentiate into respective neural precursor cells. In this context, it is essential to develop an in vivo imaging protocol for homing and tracking transplanted stem cells and to evaluate their differentiation potential at injury site.Aim and Objectives
1. Longitudinal quantitative evaluation of T2* time at injury site using absolute bias correction up to 21 days, after transplantation of iron oxide labelled MSCs.
2. To investigate the differentiation potential of infused MSCs into neuronal cells by using different neuronal specific markers (GFAP, MAP2 and NeuN) by immunohistochemistry.
Materials and Methods
All experimental procedures were conducted under the approval of the animal ethics committee of the Institute. Forty mice were investigated for MRI study at pre‑injury, after-injury and at other different time points, after MSCs transplantation in the same mice. It was divided into four groups namely, control group (10 mice, without injury), TBI group (10 mice, injured mice injected with PBS), unlabelled MSCs transplanted group (10 mice, MSCs without labelling with) and labelled MSCs transplanted group (10 mice, MSCs labelled with iron oxide). After MRI assessment, this group was investigated for differentiation potential of infused MSCs.
MSCs were isolated from bone marrow of mice and labelled with iron oxide-poly-L-lysine (IO-PLL) complex at a ratio of 50:1.5 µg/ml with an incubation period of 6 hr.3 After washing, the labelled MSCs were further labelled with red fluorescent PKH26.4 MSCs were (1.5 x106) transplanted in respective group and 7T MRI (Bruker Biospec USR 70/30, AVANCE III) was carried out up to 21 days. T2* maps were acquired using MGE_T2* sequence (TR: 1500ms; 12 echos TE/echo spacing: 4.50-65.45ms/5.54ms; matrix size: 256/192; Flip Angle: 30o, field of view: 2.5cm). The T2* values were analyzed during post-processing by performing absolute bias correction as specified in Bruker’s ISA tool4.
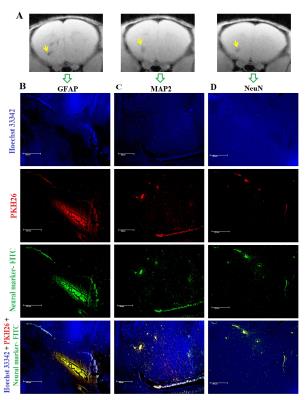
Neuronal nuclear antigen like NeuN (for neurons), MAP2 (for neuronal differentiation) and GFAP (for astrocytes) were incubated in brain sections as cell-type-specific antibodies. Sections were further incubated in Hoechst 33342 and fluorescent microscopy (PKH26: Ex/Em-551/567nm; Hoechst 33342: Ex/Em-361/497nm; FITC-antibody: Ex/Em:490/525nm) to visualise MSCs proliferation.
Results and Discussion
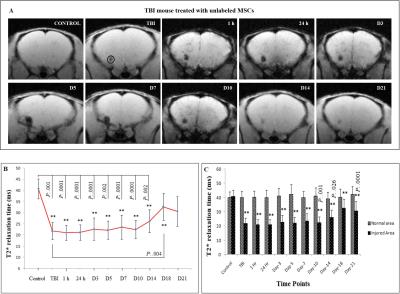
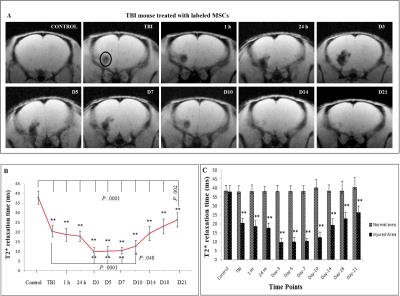
T2* time was significantly decreased at injury site after TBI (21.82 ± 3.89 ms, p < 0.001), as compared to control group (40.75 ± 4.51 ms). No major alteration in T2* time were measured after injury at respective time points in TBI group. In Fig.1, the values were found to be significantly reduced after TBI as compared to control and continued up to D14 (51.6% after 1 h, p < 0.001; 51.9% after 24 h, p < 0.001; 55.5% on D3, p < 0.001; 54.5% on D5, p < 0.001; 57.8% on D7, p < 0.001; 55.2% on D10, p < 0.001 and 64.3% on D14, p < 0.001). In Fig.2, significant decrease in T2* times were obtained in all the time points (p < 0.0001) as compared to control, whereas the relaxation times were decreased at D3 (25.7%, p < 0.0001), D5 (26.7%, p < 0.0001), D7 (27.3%, p < 0.0001) and D10 (33.2%, p < 0.048) after comparing with TBI (53.7%). The remaining time points were statistically insignificant after comparing with TBI and the values approached the baseline. Significantly less relaxation time were obtained in labelled MSCs treated group at D3 up to D10 (p < 0.0001) as compared to other groups.
IHC based fluorescent microscopy demonstrated that clearly visible neuronal differentiations of implanted MSCs were observed in MSCs infused TBI mice (Fig.3). Infused MSCs (PKH26-red) were differentiated into neuronal cells, which were demonstrated by using different neuronal markers like GFAP, MAP2 and NeuN at the site of injury.
The ascending pattern of relaxation time after D14 and neurogenic expression of transplanted MSCs, indicates tissue repair. The decline of negative signal intensity in T2*-weighted images and presence of more numbers of MSCs at injury site may provide an efficient regenerative response.
Conclusion
The serial T2* relaxation time measurement in association with absolute bias correction demonstrated that the maximum numbers of stem cells homed at the injured site on D3. The transplanted MSCs might undergo certain extent of in situ proliferation, and they appear to differentiate into neural precursors at the injury site.Acknowledgements
The project (INM-311) was financially supported by DRDO, Ministry of Defence, Govt. of India and fellowship supported from ICMR is gratefully acknowledged.References
1. Jain K K. Neuroprotection in traumatic brain injury. Drug Discov. Today 2008; 13: 1082-1089.
2. Bernard M E and Fibbe W E. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell stem cell 2013; 13: 392-402.
3. Mishra S K, Khushu S and Gangenahalli G. Potential stem cell labeling ability of poly-L-lysine complexed to ultrasmall iron oxide contrast agent: An optimization and relaxometry study. Exp Cell Res 2015; 339: 427-436.
4. Mishra S K, Rana P, Khushu S, Gangenahalli G. Therapeutic prospective of infused allogenic cultured MSCs in traumatic brain injury mice: A longitudinal proton magnetic resonance spectroscopy assessment. Stem Cells Transl Med. 2016; 5: 1-16.
Figures