3595
Detecting neuronal activity in rat model of dementia with Lewy bodies by using MEMRI1Department of Medical Imaging and Radiological Sciences, Chung Shan Medical University, Taichung, Taiwan, 2Department of Psychology, Chung Shan Medical University, Taichung, Taiwan, 3Department of Medical Imaging, Chung Shan Medical University Hospital, Taichung, Taiwan
Synopsis
Dementia with Lewy bodies (DLB) is thought to be the second commonest cause of neurodegenerative dementia in older people, accounting 15 to 25% cases at autopsy. However, to date there are no reliable methods of instrumental and laboratory diagnosis of this disease while its treatment is based on reducing the symptoms. The drug X has been shown to have neuroprotective effects in neurodegenerative diseases. Therefore, we aimed to clarify the medicinal effectiveness of drug X through manganese-enhanced magnetic resonance imaging (MEMRI). Our results indicated that drug X may have clinical potential in the treatment of DLB.
Introduction
Dementia with Lewy bodies (DLB) is thought to be the second commonest cause of neurodegenerative dementia in older people, accounting 15 to 25% cases at autopsy 1. However, to date there are no reliable methods of instrumental and laboratory diagnosis of this disease while its treatment is based on reducing the symptoms. The drug X was reported to reduce glutamatergic activity, neurotoxicity and inflammation, to influence the expression and pathological aggregation of the proteins causing neurodegeneration and to improve cognitive deficits and neurodegenerative alterations. Therefore, we aimed to clarify the medicinal effectiveness of drug X through manganese-enhanced magnetic resonance imaging (MEMRI). MEMRI is a powerful tool for detecting neuronal activities in the brain of living animals and manganese ions (Mn2+) accumulated in brain regions can be quantified by measuring the absolute T1 (or R1=1/T1) value 2,3.Materials and Methods
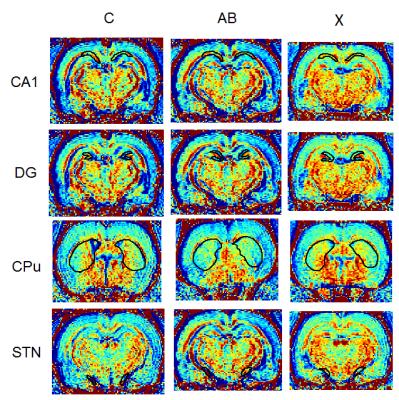
The animals were divided into three groups, 5 sham-operated rats as control group, 5 DLB rats (amyloid-β, Aβ) and 6 DLB rats were i.p. injected drug X for 14 days. They were then injected with MnCl2 (40 mg/kg, i.p.) on day 13 and underwent a brain MRI scan on day 14. Scans were performed on a 7-Tesla MRI (Bruker BioSpec, Karlshruhe, Germany). To improve detection sensitivity of Mn2+ concentrations, R1 mappings were obtained by using a spin-echo saturation recovery sequence named the rapid acquisition with relaxation enhancement with variable time of repetition (RARE-VTR). The imaging parameters of RARE-VTR sequence are: 6 TR (500, 750, 1000, 1500, 2500, and 3500 ms) and TE=8.5 ms. We measured R1 value through choosing regions of interest (ROIs). ROIs were manually defined in striatum (caudate, putamen (CPu)), subthalamic nucleus (STN), substantia nigra pars compacta (SNc), dentate gyrus (DG) and hippocampus in the coronal images. Then calculate the mean signal intensity for all voxels in the ROIs, average for both hemispheres to compare R1 differences between the groups.Results and Discussion
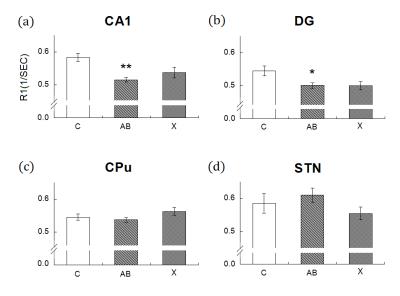
In Fig. 1 and 2, compares to control group, the rats with DLBs (Aβ) showed decreased neuronal activities in field CA1 of hippocampus (CA1), DG, CPu but showed increased activities in STN. However, only in CA1 and DG have evident decreasing. The statistical significances of CPu and STN were not reached. The treatment group compares with DLBs (Aβ) group, in CA1 and CPu, the neuronal activities showed the increase, which means the neuronal activities recovered due to injected the drug X. Hence, drug X may have the effectiveness for curing DLB. To our knowledge, DLB has many similar clinical and pathological characteristics that occurs during the course of Parkinson's disease (PD) 1. The drug X reported to cure PD may therefore have clinical potential in the treatment of DLB.Conclusion
Our results showed significantly decreased neuronal activities of DLB rats in CA1 and DG compared to control group, and the neuronal activities recovered due to injected the drug X. Our results indicated MEMRI R1 value may serve as a good indicator for DLB severity and the effect of treatment, and drug X may therefore have clinical potential in the treatment of DLB.Acknowledgements
This study was supported in part by the research programs MOST103-2410-H-040-002-MY2 and MOST104-2923-H-040-001-MY3, which were sponsored by the Ministry of Science and Technology, Taipei, Taiwan.References
1. McKeith I, et al. Dementia with Lewy bodies. Neurology. 2004; 3: 19-28.
2. Massaad CA, Pautler RG. Manganese-enhanced magnetic resonance imaging (MEMRI). Methods in Molecular Biology. 2011; 711: 145-174.
3. Eschenko O, et al. Mapping of functional brain activity in freely behaving rats during voluntary running using manganese-enhanced MRI: implication for longitudinal studies. Neuroimage. 2009; 49(3): 2544-2555.
Figures