3594
Leucine deprivation causes hypothalamic neuronal inhibition accompanied by systemic metabolic changes.1Laboratory of Molecular Imaging, Singapore Bioimaging Consortium, A*STAR, Singapore, Singapore, 2Clinical Nutrition Research Centre, Singapore Institute for Clinical Sciences, A*STAR, Singapore, Singapore
Synopsis
The
lack of dietary essential amino acid leucine influences energy metabolism via
hypothalamic leptin signaling pathway. However, these neural circuits and its
relationship with pathogenesis of obesity still remain unclear. In this work we
investigated the hypothalamic neural pathway, abdominal fat and liver fat by
multi-parametric imaging in leucine deficient diet fed mice. MEMRI results
indicated that leu deficiency triggers neuronal inhibition in certain
hypothalamic regions suggesting POMC neuronal pathway involvement in enhanced
energy expenditure through leptin signaling pathway. Combined with systemic
metabolic changes this may facilitate in understanding of amino acid sensing
and metabolic regulatory network of energy homeostasis.
Purpose
There is a growing interest in designing nutritional approaches to
regulate energy metabolism to prevent obesity and related metabolic disorders. A
strong link has been identified between amino
acid levels and frequency of metabolic diseases including obesity and type 2 diabetes 1. The dietary deficiency of the essential amino
acid leucine increases energy expenditure, activates lipolysis and enhances
insulin sensitivity 2. Leucine plays a
role in the regulation of energy expenditure via CREB/TRH pathway in the
central nervous system 3. The obvious link
between leucine deficiency and hypothalamic leptin signaling pathway suggests
that brain plays a central role in sensing of amino acid and regulation of
energy homeostasis. However, neural brain networks involved in this regulation
and its relationship with pathogenesis of obesity still remain unclear. In
this work we investigated the hypothalamic neural pathway, abdominal fat and
liver fat by multi-parametric imaging in leucine deficient diet fed mice.Methods
Animal experiments were approved by the instutional animal care and use committee (BRC, ASTAR, Singapore). The C57BL/6 (male, 12-16 weeks old) mice were randomized into two cohorts fed with different diets including control or leucine-deficient ((-)leu) diets for 14 days. First, we measured Manganese-Enhanced MRI (MEMRI) signal (MnCl2, 5ml/Kg, 62.3 mM, i.v.) after 14 days of leu(-) or control diet intervention using a 9.4T scanner (Biospec, Bruker BioSpin GmbH, Germany) with 72mm volume coil and mouse brain array. T1-weighted images were acquired using MDEFT sequence with TR/TE/TI = 8/2.55/1000, flip angle = 10°, FOV = 19.2 × 19.2 × 25 mm, matrix size = 192 × 192 × 50 with acquisition time of 2.3min/time frame for 180 mins. Body composition imaging was performed using a 7T ClinScan scanner (Bruker) with a mouse body coil for abdominal imaging and a volume transmit / surface receive coil for liver MRS. Dixon imaging was performed to acquire water and fat images from abdomen region (lumbar I to V) with TR= 8, 24 slices, TE (opposite phase) = 1, TE (in phase) = 2.5, flip angle = 8°. Segmentation of subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT) was performed using a hybrid algorithm by an in-house developed MATLAB program. Liver spectroscopy was performed by volume localized PRESS sequence with TR/TE = 4s/13ms, NA 64, voxel volume 16 mm3, SW = 3500 Hz, data points = 2048. Fat content from in vivo spectra was estimated using LC Model software.Results
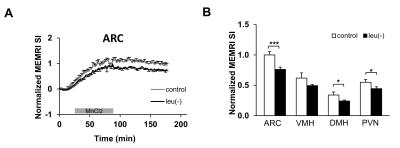
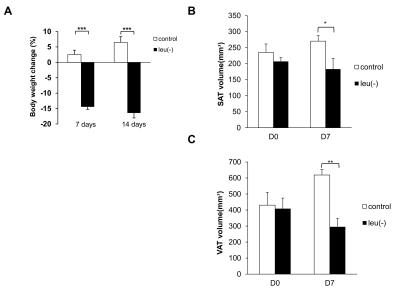
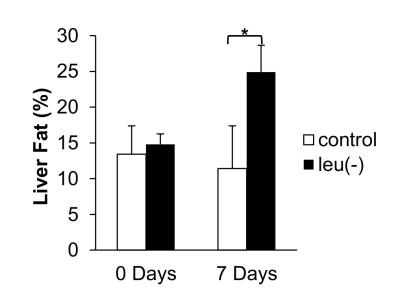
Figure 1A shows the comparison of time course measurements of MEMRI signal intensity (SI) in ARC (arcuate nucleus) of the brain in mice fed with leu (-) vs control diet. There is a reduction in MEMRI signal in leu(-) compared to control group. Moreover, there is a significant reduction of MEMRI SI in DMH (dorsomedial nucleus) and PVN (paraventricular nucleus) (Fig. 1B). The body weight reduced by 14% and 16% after 7 and 14 days respectively in leu(-) diet group compared to control group (Fig. 2A). The VAT reduced by 40%, whereas there was no change in SAT after 7 days of leu(-) diet (Fig.2 B-C). The hepatic fat content increased by 70% in in leu(-) group compared to control group (Fig. 3).Discussion
MEMRI signal is one of the ways to map activated neuronal pathway in the hypothalamus in vivo. Therefore, decreased MEMRI SI in ARC region of leucine-deprived mice suggests suppression of neuronal firing, i.e. lower activity of voltage-gated Ca2+ channels resulted in decrease of Mn2+ entry and accumulation. Our results are in agreement with earlier work which showed that mice fed with leucine-deprived diet have lower leptin levels and as result lower POMC expression 4. Additionally, lower MEMRI SI in DMH and PVN apart of ARC suggest involvement of these hypothalamic regions in leucine deprivation signaling pathway. In additon, large decrease of body weight is due to the loss of VAT implying increased lipolysis in white adipose tissue. Similarly, increase of fat in liver after 7 days of leucine-deprived diet together with mobilization of lipids stored in adipose tissue may be an indicator of metabolic adaptation caused by protein deprivation.Conclusion
MEMRI results indicated that leu deficiency triggers overall neuronal inhibition in several hypothalamic regions during basal non fasted condition, which in turn suggests that POMC neuronal pathway may be responsible for enhanced energy expenditure through leptin signaling pathway. Combined with systemic metabolic changes this may facilitate in understanding of amino acid sensing and metabolic regulatory network of energy homeostasis.Acknowledgements
The work was supported by the Singapore-China Joint Research Programme - 10th Joint Grant Call ID1215c Project ID1418324005.References
1. Lynch CJ, Adams SH. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat Rev Endocrinol 2014;10:723-736.
2. Cheng Y, Meng Q, Wang C, et al. Leucine deprivation decreases fat mass by stimulation of lipolysis in white adipose tissue and upregulation of uncoupling protein 1 (UCP1) in brown adipose tissue. Diabetes 2010;59:17-25.
3. Xia T, Zhang Q, Xiao Y, et al. CREB/TRH pathway in the central nervous system regulates energy expenditure in response to deprivation of an essential amino acid. Int J Obes (Lond) 2015;39:105-113.
4. Zhang Q, Liu B, Cheng Y, et al. Leptin signaling is required for leucine deprivation-enhanced energy expenditure. J Biol Chem 2014;289:1779-1787.