3587
Multi-parametric 11C-Methionine-PET/MRI for brain tumor imaging utilizing MR Fingerprinting.1Department of Diagnostic and Interventional Radiology and Neuroradiology, University Hospital Essen, Essen, Germany, 2Siemens Healthcare GmbH, Erlangen, Germany, 3University Duisburg-Essen, Erwin L. Hahn Institute for MR Imaging, Essen, Germany, 4High Field and Hybrid MR Imaging, University Hospital Essen, Germany, 5Case Western Reserve University, Cleveland, OH, 6Department of Nuclear Medicine, University Hospital Essen, Essen, Germany
Synopsis
The successful implementation of integrated PET/MR systems has enabled a unique platform for simultaneous multi-parametric imaging comprising morphologic, functional and metabolic features of pathologic tissue. MR Fingerprinting has been recently presented as a robust and fast framework for simultaneous accurate quantification of multiple MR tissue properties. The results of our study, combining tissue characterization based on PET/MR imaging and MR Fingerprinting, indicate a correlation between tumor grading and changes in tissue features, demonstrating the high diagnostic potential of this novel approach for multi-parametric tissue characterization.
Introduction
11C-Methionine PET imaging has been shown to provide a high detection rate of brain tumors as well as improved assessment of potential tumor recurrences. With the successful introduction of integrated PET/MR systems into scientific and clinical imaging, a new platform for simultaneous multiparametric assessment of morphologic, functional and metabolic features of brain tumors has been established (1). As an additional parametric feature, in-vivo relaxometry offers valuable information on tissue characterization. Magnetic Resonance Fingerprinting (MRF) has been shown to provide simultaneous quantification of multiple tissue properties, with initial demonstration in relaxometry (2,3). The aim of this study was to investigate a novel approach of multiparametric tissue characterization of brain tumors based on morphologic, functional, metabolic and relaxometric properties, pulling together quantitative data from different imaging modalities.Methods
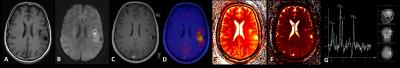
Imaging: 10 patients (2 f, 3 m; mean age 45.2, age range 25-62) with suspected brain tumors or suspected tumor relapse were enrolled in this trial. 8 of these 10 patients showed tumor / tumor relapse, 2 post-therapeutic changes. Examinations were performed on an integrated 3-Tesla PET/MR system (Biograph mMR, Siemens Healthcare, Erlangen, Germany). PET and MR imaging was obtained simultaneously in one bed position (20 minutes) after injection of 11C-Methionine (mean ± SD, 752.1 MBq; ± 254.2 MBq). The (PET)MRI protocol included the following sequences: (1) T1-weighted TIRM (repetition time [TR], 2000 ms; echo time [TE], 13 ms; slice thickness (ST), 5 mm), (2) 3D-FLAIR (TR 5000 ms, TE 395 ms, ST 1 mm), (3) diffusion-weighted imaging (TR 7900 ms; TE 101 ms; b-values: 0,1.000 s/mm2 ; ST 5mm), (4) susceptibility-weighted imaging (TR 26 ms, TE 20 ms, ST 2 mm), (5) post contrast 3D-MPRAGE imaging (0.5 mmol/kg bw gadoterate meglumine; Dotarem, Guerbet) (TR 1790 ms, TE 2.67 ms, ST 1 mm), (6) single-voxel spectroscopy (TR 2000 ms, TE 135 ms, ST 15 x 15 x15mm3). MR Fingerprinting was performed on a 3-Tesla MR scanner (MAGNETOM Skyra, Siemens Healthcare) utilizing a prototype implementation of the MRF technique (1). Imaging was acquired through the representative solid areas of the tumors, and quantitative T1 and T2 maps were generated. Multiple regions of interest (ROIs) were manually outlined at predetermined locations of the tumors, and T1 and T2 relaxation values at these sites were extracted. Each slice acquisition amounted to a total of approx. 40 seconds. Evaluation: Data analysis was performed by an experienced radiologist and a nuclear medicine physician. To quantify the metabolic activity of 11C-Methionine-avid lesions, maximum standardized uptake values (SUVmax) were determined by drawing a 3-dimensional isocontour on PET/MR images. In addition, the ratio to uptake in normal brain parenchyma of the contralateral normal cortex was calculated (T/N ratio). ADC values were calculated based on ROI analysis. Relaxation times of the suspicious lesions were measured by manually placing regions of interest in the calculated parametric maps.Results
The following tumor entities were detected in the investigated patient cohort: (1) primary low-grade glioma grade 2 (n=1), (2) relapse of a low-grade glioma grade 2 (n=1), (3) primary astrocytoma grade 3 (n=1), (4) multifocal astrocytoma grade 3-4 (n=1), (5) primary glioblastoma (n=3), (6) relapse of glioblastoma (n=1). An increase in malignancy from low-grade (grade 1-2) to high-grade glioma (grade 3,4) was associated with increasing / positive PET uptake, contrast enhancement, increasing values in Choline and Choline/Creatine as well as increasing T1 and T2 values. While low-grade astrocytoma did not show any pathologic tracer uptake or contrast enhancement, grade 3 gliomas showed increasing tracer uptake and increasing T1 and T2 values in MRF, respectively (mean values grade 3 tumors: SUVmax:1.8 T/N: 1.3; T1: 1442/ T2: 122.9; mean values grade 4 tumors: SUVmax: 3.4 T/N: 2.1; T1: 1752/ T2: 93.8). In one patient, the diagnosis based solely on the MRI data was altered from low-grade astrocytoma (grade 2; no contrast enhancement) to high-grade astrocytoma (grade 3) due to PET uptake, showing correspondingly increased T1 and T2 relaxation values.Discussion & Conclusion
This study presents preliminary results of an enhanced multi-parametric approach to tissue characterization of brain tumors. The results of this novel approach of multi-parametric PET/MR imaging of brain tumors including MR Fingerprinting demonstrate corresponding changes in tissue property based on the combined assessment of morphologic, functional, metabolic and relaxometric properties, indicating a correlation to the grading of brain tumors and leveraging tissue characterization to a unique level of multi-parametric assessment.Acknowledgements
No acknowledgement found.References
1. Boss A, Bisdas S, Kolb A, Hofmann M, Ernemann U, Claussen CD, et al. Hybrid PET/MRI of intracranial masses: initial experiences and comparison to PET/CT. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2010;51(8):1198-205.
2. Ma D, Gulani V, Seiberlich N, Liu K, Sunshine JL, Duerk JL, Griswold MA.Magnetic resonance finger printing. Nature. 2013 Mar 14;495(7440):187-92
3. Jiang Y, Ma D, Seiberlich N, Gulani V, Griswold MA. MR fingerprinting using fast imaging with steady state precession (FISP) with spiral readout. Magn Reson Med. 2015 Dec;74
Figures