3586
Aza-BODIPY Derivative for Gadolinium-enhanced MR and Near-infrared Fluorescence Dual Imaging1Bio-imaging Research Team, Korea Basic Science Institute, Cheongju, Korea, Republic of, 2Graduate School of Analytical Science and Technology, Chungnam National University
Synopsis
A novel dual imaging probe for in vivo magnetic resonance imaging (MRI) and optical imaging was developed by combining gadolinium (Gd)-chelating MR probe and a near-infrared (NIR) fluorophore, aza-BODIPY (AB; BODIPY = borondipyrromethene). This aza-BODIPY-based bimodal contrast agent (AB-BCA) showed a significant fluorescence emission around the NIR range and an enhanced longitudinal relaxivity in MR modality. The probe was easily delivered to phagocytic cells of the innate immune system, together with macrophages and dendritic cells (DCs), and presented high-performance fluorescence and MR imaging without obvious cytotoxicity.
Introduction
Nanoparticle-based imaging contrast agents have been mainly evaluated as carriers of MRI contrast and fluorescent agents for noninvasive monitoring of immune cells in vivo. Bimodal probes incorporated with MRI contrast agents and quantum dot (QD) have also been used for immune cell tracking and imaging. However, the potential toxicity of QD precluded its extensive use in clinical applications. Although various bimodal contrast agents using organic dyes have been widely reported, borondipyrromethene (BODIPY)-based contrast agents are rarely used for dual imaging modalities. BODIPY derivatives are promising optical probes with high stability, sharp emission bands, high extinction coeffiicients, and high quantum yields.Purpose
We report a new bimodal contrast agent that allows targeted labeling and in vivo tracking of immune cells such as macrophages and DCs. We synthesized an aza-BODIPY-based contrast agent (AB-BCA) possessing two gadolinium chelates for NIR and MR imaging.Methods
Preparation of Gd3+ Complex. The ligand (AB-BCA, 90 μM) was in ultrapure water (10 mL), and the solution was adjusted to pH 7 with sodium bicarbonate. Gadolinium chloride hexahydrate (170 μM) was dissolved in 3.0 mL of ultrapure water and added to the ligand solution (AB-BCA) in three separate aliquots.
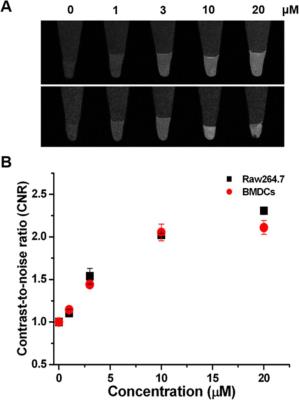
Cell Viability Assay and Cellular Imaging. The cytotoxicity of AB-BCA was measured by performing 3-(4,5- dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Roche Diagnostics GmbH, Mannheim, Germany) assay on RAW 264.7 cells and BMDCs. Cells were seeded in a 96-well cell culture plate at 1 × 104 cells per well under a humidified atmosphere containing 5% CO2 at 37 °C for 24 h. Fluorescence microscopy images were acquired using a laser scanning confocal microscope (LSM 710, Carl Zeiss, Germany). T1-weighted MR images of the cell phantoms with spin−echo pulse sequence were measured from the RAW 264.7 cells and BMDCs labeled with AB-BCA incubated with different concentrations of AB-BCA using a 4.7 T MRI scanner. The following MRI parameters were used: field of view (FOV) = 5 × 3 cm2, matrix size = 256 × 256, slice thickness = 1 mm, echo time (TE) = 10 ms, and repetition time (TR) = 350 ms.
Results
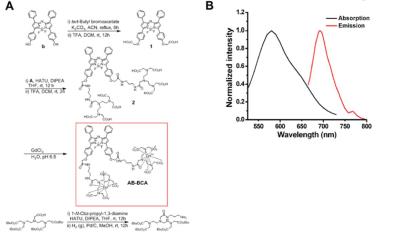
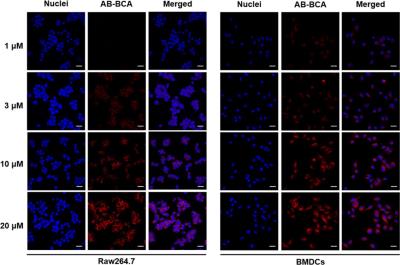
AB-BCA showed a maximum absorption at 580 nm (Figure 1), and its emission exhibited a maximum peak at 690 nm around the range of NIR. Therefore, NIR fluorescence-based optical imaging using AB-BCA can be used to facilitate the cell detection and tracking. Before being applied to the biological milieu as a contrast agent, the relaxivity (r1) values at 60 and 200 MHz were measured as 13.3 ± 0.1 and 3.9 ± 0.1 mM−1 s−1, respectively, which is 3.2-fold higher at 60 MHz than the r1 value of the clinically available T1 contrast agent, Gd-DTPA-BMA. In the optical imaging study, the fluorescence signal started to be visible in the cytoplasm of Raw 264.7 cells and BMDCs at 1 h after treatment with AB-BCA. NIR fluorescence was increased after 2 h of incubation and maximized after 4 h in both cell types. The fluorescence intensities after incubation for 4 h (Figure 2) indicated that the fluorescence in the AB-BCA-treated cells was gradually increased and saturated after treatment with 10 μM of AB-BCA, which means the cellular uptake of AB-BCA was concentration-dependent.Discussion and Conclusion
We synthesized the NIR-emitting aza-BODIPY with Gdchelating units at the two arms of the fluorophore. The ABBCA showed sufficient relaxivity to enable effective signal enhancement in MRI and NIR fluorescence-enhanced optical imaging and low cytotoxicity on innate immune cells, allowing continuous monitoring of labeled cells in vivo. In addition, ABBCA, as an optical/MR dual imaging agent, can easily be used to label the intracellular regions of macrophages and BMDCs, supporting high-performance MR and fluorescence imaging.Acknowledgements
No acknowledgement found.References
1. Kurtz, D. M.; Gambhir, S. S. Tracking Cellular and Immune Therapies in Cancer. Adv. Cancer Res. 2014, 124, 257−296.
2. Kircher, M. F.; Gambhir, S. S.; Grimm, J. Noninvasive Celltracking Methods. Nat. Rev. Clin. Oncol. 2011, 8, 677−688.
Figures