3583
MR/optical image-guided liposomal drug delivery system for triple negative breast cancer1Radiology and Radiological Science, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2Life Science Tokyo Advanced Research Center (L-StaR), Hoshi University School of Pharmacy and Pharmaceutical Science, Tokyo, Japan, 3SibTech, Inc., Brookfield, CT, 4CadenzaMed LLC, Wayne, PA, 5Department of Oncology, The Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, Baltimore, MD, United States
Synopsis
Noninvasive image-guided drug delivery is important in cancer drug development, and allows visualizing the delivery and predicting the outcome. In this study, the delivery and therapeutic effects of liposomes targeted to tumor vasculature was researched. Intravital microscopy and MRI were used for tracking the drug delivery and therapeutic effects were monitored in MDA-MB-231 mouse tumor model. Our experimental results suggest that targeting liposomes to VEGF receptors (VEGFRs) expressed on tumor endothelium, may inhibit EPR-mediated extravasation and accumulation of VEGFR-targeted liposomes.
Introduction
Noninvasive image-guided nanocarrier platforms are highly important in cancer drug development, and allow visualizing the delivery and thus predicting the outcome. Many tumors including triple-negative breast cancer (TNBC) do not have established molecular targets and in this case targeting of tumor vasculature, which expresses unique molecular markers, can be a valuable option.1 In this study, we investigated the specific therapy by paclitaxel loaded liposomes targeted to vascular endothelial growth factor receptors (VEGFRs) overexpressed in tumor vasculature. MRI and optical image guidance was used to characterize the delivery of liposomes in animal models of TNBC.Methods
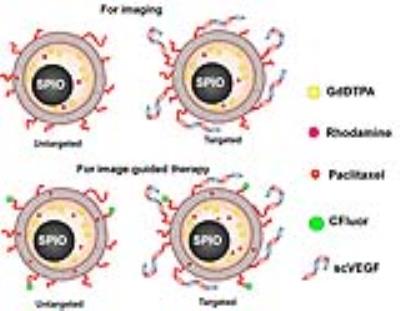
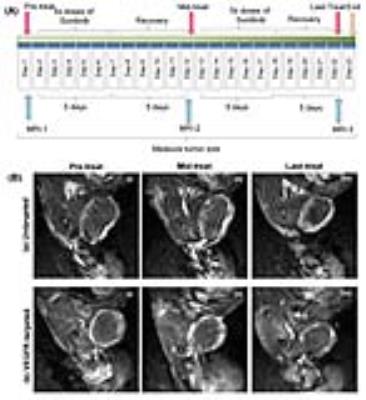
Liposomal nanocarriers were formulated using DSPE lipid and cholesterol, labeled with Ominiscan or nanomag-D-spio magnetic nanoparticles, and loaded with paclitaxel. Liposomes were also labeled with rhodamine (Rhod) or DSPE-PEG2-CFluor fluorescent markers. For targeting the liposomes were decorated with single-chain VEGF (scVEGF) by post-insertion of scVEGF-PEG3400-DSPE conjugates into the lipid bylayer (Figure 1). Orthotopic MDA-MB-231 TNBC models were grown in the mammary fat pad of athymic mice. MR studies were performed on a 9.4 T Bruker Biospec spectrometer. T1- and T2-weighted images were acquired using the RARE sequence before and after the treatment with liposomes at a dose of 11 μmol Fe/kg to assess the delivery and stability of liposomes loaded with both OminiscanTM and nanomag®-D-spio.2 Gd-DTPA enhanced mages were acquired with a 3D gradient T1-weighted echo sequence, using TE/TR = 2/6 ms, and flip angle 23 degree. For intravital multiphoton fluorescence microscopy (MPM) a "U" shape skin-flap was cut in the dorso-lateral skin of the tumor and the animal was immobilized on the mouse holder. Mice were i.v. injected with targeted Lip(scVEGF)(Gd/Fe)(Rhod) or untargeted Lip(Gd/Fe)(Rhod) liposomes, respectively, and fluorescence images were taken using an Olympus FV1000MPE multiphoton microscope (Figure 3). For the therapeutic study, Lip(scVEGF)(Gd/Fe)(Px)(CFluor) and Lip(Gd/Fe)(CFluor)(Px) were used. Two groups of mice were treated orally with sunitinib (5 days on/5 days off) and therapeutic liposomes at days 1, 12, and 21 (Figure 4A). Animals in the control group received saline injections. MRI was performed at days 1, 12, and 21 (Figure 4B). Tumors and organs were extracted for the determination of biodistribution of paclitaxel. Dimensions of tumors were measured by a caliper every 3rd day and by principal axes determined from the MRI images on day 1, 12, and 21. The paclitaxel content in liposomes and paclitaxel biodistribution were measured by HPLC.Results
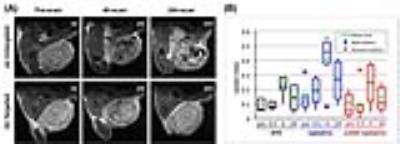
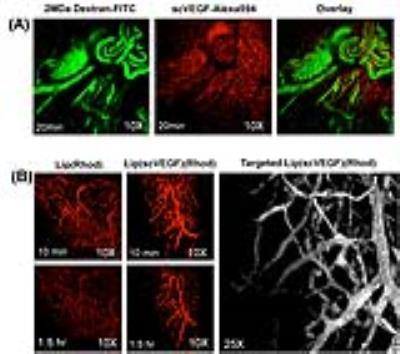
The T2-weighted MR images of tumors before and after i.v. administration of untargeted- and targeted-liposomes are shown in Figure 2. These results revealed that at 4 and 24 hours post-injection untargeted-liposome provided improved tumor accumulation compared to the VEGFR-targeted liposomes. The highest uptake index occurred 4 h after the administration in all three groups; however, only Lipo(Gd/Fe) produced statistically significant hypo-intense tumor signals at 4 h time point (Figure 2). Intravital MPM images of the distribution of the Lip(Rhod) in the tumor vasculature are shown in Figure 3A. Complete clearance of the probe from the blood stream and binding to the blood vessel walls was detected 35 min after administration. MPM images of untargeted and scVEGF-targeted Lip(Rhod) in the vasculature of MDA-MB-231 tumors are shown in Figure 3B. High magnification images (left) demonstrate extravasation of the untargeted liposomes due to EPR effect whereas no extravasation was detected for the targeted liposomes presumably due to high-affinity binding to the endothelial cells via VEGFR2 receptors. The results of ongoing studies demonstrate minimal therapeutic effects measured form changes in tumor volumes and biodistribution of paclitaxel measured by HPLC between the Lip(scVEGF)(Gd/Fe)(Px)(CFluor) and Lip(Gd/Fe)(Px)(CFluor) following the treatment plan in Figure 4A. Gd-DTPA enhanced MRI images demonstrated similar tumor enhancement pattern in mice received untargeted and targeted liposome (Figure 4B).Discussion
Efficient delivery of therapeutic liposomes was detected by MRI and optical imaging. However, no increase in the delivery was detected for liposomes specifically targeted to tumor vasculature. We hypothesize that binding of liposomes to VEGFRs on tumor endothelium, may even inhibits their intratumoral delivery via non-specific EPR-mediated extravasation and accumulation. However, we also cannot exclude the possibility that decoration of liposomes with scVEGF could by itself change their pharmacokinetics and the ability to extravasate. In addition, sub-therapeutic doses of paclitaxel loaded liposomes were used, which might affect treatment outcomes.Conclusions
Noninvasive MRI and intravital microscopy can provide critical information for delivery of nanomedicine and for tracking of delivery and therapeutic effects of these nano-constructs in preclinical cancer models. The concept of vascular targeting is still in its infancy and requires significantly more rigorous research and development using image-guided approach in appropriate animal models.Acknowledgements
This study was supported by the National Institutes of Health, CA154738 (DA, JMB, and SS). We are grateful to Mr. Desmond Jacob for technical assistance in multiphoton fluorescence microscopy.References
1. Onuki Y, Jacobs I, Artemov D, et al. Noninvasive visualization of in vivo release and intratumoral distribution of surrogate MR contrast agent using the dual MR contrast technique. Biomaterials 2010;31(27):7132-7138.
2. Kato Y, Zhu W, Backer MV, et al. Noninvasive imaging of liposomal Delivery of superparamagnetic iron oxide nanoparticles to orthotopic human breast tumor in mice. Pharmaceut Res. 2015;32(11):3746-3755.
Figures