3581
MR Imaging of Nanoparticle Uptake and 3D Intra-tumoral Distribution in Orthotopic Xenograft Mouse Models of Neuroblastoma1Pediatric Radiology, Texas Children's Hospital, Houston, TX, United States
Synopsis
Heterogeneity in tumor vasculature gives rise to variability in vascular permeability, one of the consequences being non-uniform intra-tumoral uptake and distribution of nanoparticle-based chemotherapeutics. An imaging agent that can assess tumor vascular heterogeneity and monitor uptake and distribution of nanoparticles could be useful for guiding and monitoring nanoparticle-based chemotherapy. In this work, we demonstrate, in orthotopic mouse models of solid tumors, that a high T1 relaxivity liposomal-gadolinium contrast agent (liposomal-Gd) enables MR imaging of nanoparticle uptake and 3D intra-tumoral distribution at clinically-relevant MR field strength.
Introduction
Nanoparticles exhibit heterogeneous uptake and distribution in solid tumors. Non-invasive imaging techniques that can monitor uptake and intra-tumoral distribution of nanoparticles are of interest for personalized nano-medicine1. In this work, we investigate a high T1 relaxivity liposomal-gadolinium contrast agent (liposomal-Gd) for MR imaging of nanoparticle uptake and distribution in orthotopic mouse models of solid tumors.Methods
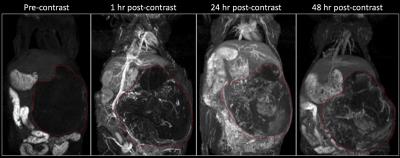
In vivo studies were performed in two orthotopic mouse models of pediatric neuroblastoma (NB)2. Human NB cell lines (NGP: a MYCN-amplified and SH-SY5Y: a MYCN non-amplified) were surgically implanted beneath the renal capsule of nude mice. MR imaging was performed on a small animal 1T permanent magnet. Tumor growth was monitored using T2-weighted (T2w) fast-spin echo (FSE) imaging. Dynamic contrast-enhanced MRI was performed using a T1-weighted gradient echo sequence (T1w-GRE). A high T1 relaxivity liposomal-Gd contrast agent (0.1 mmol Gd/kg) was used as a surrogate for monitoring the uptake and intratumoral distribution of nanoparticles3. Imaging was performed pre-contrast and then at 1 hr, 24 hr and 48 hr post-contrast. Image analysis was performed in pre-contrast and 48hr post-contrast images to assess spatial variation in intratumoral signal intensity. Radial analysis of signal intensity was performed to assess signal enhancement for core, intermediate and peripheral region of the tumors. Animals were euthanized after completion of imaging study and the tumors harvested for histological examination and fluorescence microscopy analysis of intra-tumoral nanoparticle distribution.Results
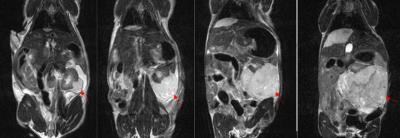
Longitudinal T2w-MRI enabled in vivo monitoring of tumor development in the implanted kidney (Figure 1). As the tumors aged, a multi-lobular growth was evident on T2w-FSE images. The long circulating property of liposomal-Gd enabled dynamic interrogation of tumor vasculature (Figure 2). Early phase imaging (1 hr post-contrast) enabled high-resolution visualization of tumor vasculature since the liposomal-Gd is predominantly distributed in the vascular compartment. Delayed-phase imaging (48 hr post-contrast) demonstrated signal enhancement in the tumor due to extravasation and accumulation of liposomes. The tumors exhibited high variability in the intra-tumoral signal enhancement, suggesting a heterogeneous pattern of nanoparticle distribution in the tumors. The signal enhancement was highest in the peripheral tumor region (268 ± 144 a.u.), suggesting the presence of highly permeable blood vessels in the expanding peripheral region. The intermediate region of the tumor core exhibited moderate enhancement (177 ± 90 a.u.); the lowest signal enhancement was observed in the core region (96 ± 56 a.u.) Fluorescence microscopy analysis of tumor tissue demonstrated the well-known perivascular distribution of nanoparticles.Conclusions
A high T1 relaxivity liposomal-Gd contrast agent enabled MR imaging of uptake and intra-tumoral 3D distribution of nanoparticles in solid tumors at clinically-relevant MR field strength. This methodology could allow for non-invasive monitoring of tumor uptake of nano-chemotherapeutics.Acknowledgements
No acknowledgement found.References
1. Prabhakar U, Maeda H, Jain RK et al., Sevick-Muraca EM, Zamboni W, Farokhzad OC, Barry ST, Gabizon A, Grodzinski P, Blakey DC. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. 2013. pp. 2412– 7.
2. Patterson DM, Shohet JM, Kim ES. Preclinical models of pediatric solid tumors (neuroblastoma) and their use in drug discovery. Curr Protoc Pharmacol. 2011 Mar;Chapter 14:Unit14.17.
3. Ghaghada KB, Ravoori M, Gnanasabapathy D et al. New Dual Mode Gadolinium Nanoparticle Contrast Agent for Magnetic Resonance Imaging. PLoS One. 2009; 4(10):e7628.
Figures