3576
In vivo CEST MRI of specific activated T-cells population using the natural nucleoside cytidine1Organic Chemistry, Weizmann Institute of Science, Rehovot, Israel
Synopsis
Non-invasive imaging of inflammation is crucial for understanding both inflammatory processes and immunotherapy. We propose a non-invasive and non-radioactive approach for longitudinal imaging of activated immune cells. We show that the use of the natural nucleoside cytidine as CEST imaging probe enable the monitoring of specific activated T-cells population with MRI. By using fluorescent analog of cytidine it is shown that CD8 T-cells are in situ labeled, underlying the specificity of nucleosides as potential probes for monitoring activated immune cells. We conclude that by using cytidine as a MRI-based probe one could monitor specific activated T-cells population during inflammation.
Introduction
Non-invasive imaging of immune cells has gained a lot of interest mainly in the context of monitoring inflammatory processes and immunotherapies. More specifically, the ability of tracking activated T-cells became relevant for understanding their stimulation and localization toward the evaluation of inflammation onset and the determination of immunotherapy efficacy. Both iron-oxide and fluorinated nanoparticles have been proposed for those purposes and extensively studied1. However, in situ labeling and imaging of specific population of activated immune cells will enable better understanding of the immune system in health and disease. Capitalizing on the fact that nucleoside kinase expression is high in proliferating lymphoid cells/tissues2 and on the accumulation of nucleoside in cells expressing high levels of nucleoside kinases 3, 4 we propose a non-invasive and non-radioactive approach for longitudinal imaging of specific activated T-cells in inflammation model. Using the natural nucleoside cytidine for CEST MRI and its fluorescent analog for flow cytometry we demonstrate the ability of in situ labeling of specific T-cells population.
Methods
SJL/JCrHsd mice were immunized with 150 μg PLP 139-151 myelin epitope emulsified in complete Freund’s adjuvant (CFA) containing 150 μg Mycobacterium tuberculosis.
CEST MRI: For in vitro experiments, 10-14 days post immunization mice were sacrificed and lymph nodes cells were extracted and incubated in the presence of both, immunogenic antigen and cytidine for the determination of cells proliferation by CEST MRI. For in vivo imaging, ten days post immunization mice were anesthetized and a series of 3 CEST data sets were obtained, i.e., before, and 10 and 60 min following intravenously injections of cytidine (500mg/kg) or PBS. CEST weighted images were acquired with a modified RARE pulse sequence (TR/TE = 5000/4.8 ms), using a 150 Hz/3000 ms saturation pulse alternating between ±3 ppm frequency offsets from the water frequency. For each time point, the MTRasym map was calculated from [S−1.8 ppm - S+1.8 ppm] /S0 following B0 correction.
FACS analysis: For the determination of cytidine uptake, 10-14 days post immunization mice were intravenously injected with fluorescent analogs of either cytidine or cytosine (Fig. 1A). Sixty min post injection, lymph nodes cells were extracted and stained with cell surface antibodies for CD4, CD8 T cells, and immediately analyzed by flow cytometry for pyrrolo-cytidine, and pyrrolo-cytosine accumulation.
Results
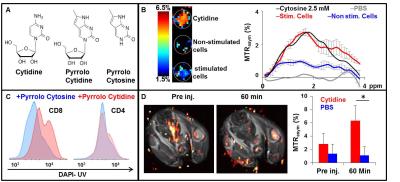
In this study we evaluated the potential of cytidine to be used as natural CEST MRI probe for the detection of specific population of activated immune cells. The chemical structures of cytidine and its fluorescent analogs are shown in Fig.1A. In vitro CEST MRI of cell lysate of antigen stimulated lymphocytes is shown in Fig.1B, depicting a more significant CEST signal in stimulated cells compared to non-stimulated cells. MTRasym plot shows the highest CEST signal of stimulated cell at Δω=1.8 ppm pointing on cytidine uptake and demonstrating antigen specific immune response using natural nucleoside cytidine. Fig. 1C shows FACS analysis of activated immune cells following injection of fluorescent analogs, demonstrating a significant uptake of cytidine analog by T-cells sub-clusters, CD8 and not by CD4 T-cells. In contrast, cytosine analog did not accumulate in immune cells following its injection, demonstrating the specificity of the nucleoside uptake rather than base-only cytosine. Fig.1D shows the MTRasym map of inflamed mouse prior and 60 min following cytidine injection. Quantitative analysis of CEST signal in the immunized lymph node region demonstrates the longitudinal changes in CEST contrast post cytidine injection. Both MTRasym maps (obtained at Δω=1.8 ppm) and plots demonstrate the accumulation of injected cytidine in the lymph node 60 min post injection. Contrary, injection of PBS did not result in any CEST effect.
Discussion
In this study, we show that the natural nucleoside cytidine could be used as a cell-specific reporter probe for CEST MRI. Cytidine uptake by stimulated immune cells was demonstrated in vitro, allowing the detection of antigen specific immune response. In vivo CEST MRI of inflamed lymphoid organs using cytidine allowed the evaluation of inflammation progression longitudinally. Both, in vitro and in vivo experiments demonstrate the potential of using natural nucleoside as biocompatible sensor for cell based proliferation setups and molecular imaging approaches in term of safety and non-radioactive properties. Furthermore, we have demonstrated the in situ labeling of specific T-cells population using fluorescent cytidine analog, underlying the specificity of nucleosides as molecular probes for monitoring activated immune cells.
Conclusions
We assessed the feasibility of using the natural nucleoside cytidine as a suitable MRI based probe for longitudinal imaging of specific population of activated T-cells during inflammation.
Acknowledgements
No acknowledgement found.References
1. Ahrens, E.T. & Bulte, J.W. Tracking immune cells in vivo using magnetic resonance imaging. Nature reviews. Immunology. 2013;(13): 755-763.
2. Arner, E.S. & Eriksson, S. Mammalian deoxyribonucleoside kinases. Pharmacology & therapeutics. 1995;(67):155-186.
3. Radu, C.G. et al. Molecular imaging of lymphoid organs and immune activation by positron emission tomography with a new [18F]-labeled 2'-deoxycytidine analog. Nature medicine. 2008(14):783-788.
4. Shields, A.F. et al. Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nature medicine.1998;(4):1334-1336.
Figures