3574
CEST MRI using sugar alcohol, maltitol, to detect cancer: Study on rat glioma modelPuneet Bagga1, Mohammad Haris2, Kevin D'Aquilla1, Francesco Marincola2, Hari Hariharan1, Ravinder Reddy1, and Puneet Bagga3
1Department of Rediology, University of Pennsylvania, Philadelphia, PA, United States, 2Research Branch, Sidra Medical and Research Center, Doha, Qatar, 3Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States
Synopsis
We show the use of a sugar alcohol sweetener, maltitol, in cancer MRI studies by exploiting its chemical exchange saturation transfer (malCEST) property. The tumor specific accumulation of maltitol in a rat glioma model provides localized, temporal changes in the CEST contrast, which corroborated the gadolinium-enhanced MRI. These findings illustrate the potential application of maltitol in the diagnosis and monitoring of the therapeutic response of cancers, including gliomas in preclinical studies.
Introduction
Chemical exchange saturation transfer (CEST) property of hydroxyl (–OH) groups on glucose and its analogs has been exploited recently to detect and image cancer in humans and animals1,2,3,4,5. Here, we describe an MRI contrast method for the detection of cancer using a sugar alcohol, maltitol, which exploits the chemical exchange saturation transfer (CEST) property of the labile –OH protons on maltitol (malCEST). In a rat gliosarcoma model, administration of maltitol leads to a clear elevation of malCEST contrast in the tumor due to the accumulation of maltitol.Material and Methods
High-resolution NMR spectroscopy of maltitol (Sigma-Aldrich, USA) solution (300 mM, pH 7) was performed on 400 MHz vertical bore scanner (Bruker, Germany) at 5 ºC with a single pulse-acquire spectroscopy with TR=4 s, number of averages=32. Solution phantoms with varying concentration and pH at 37 °C, were used to evaluate the CEST properties of maltitol at 9.4T (Agilent, USA) with following parameters: slice thickness=10 mm, GRE flip angle=5º, GRE readout TR=5.6 ms, TE=2.7 ms, FOV=20×20 mm2, matrix size=128×128, T1delay=15 s. CEST images from 0 to ±5 ppm were collected in step size of 0.2 ppm at 7 µT saturation pulse power (B1) and saturation duration 2 s. In vivo CEST MRI was performed at 9.4T MRI scanner (Agilent, USA) on rats injected with 9L glioma cells intracranially 3 weeks prior to the experiment. Tumor-bearing mice were maintained under 1-1.5% isoflurane in O2, supplied at 1 L/min. During the course of the experiment, the body temperature was maintained at 37ºC. The CEST sequence parameters were the same as the phantom study parameters except, 30×30 mm2, slice thickness=4 mm, T1delay=8s, 0 to ±3.6 ppm in the step size of 0.2 ppm at 2.34 µT saturation pulse power (B1) and saturation duration 2s. B0 correction was done by acquiring WASSR images at 0.23 μT from -1 to +1 ppm in steps of 0.1 ppm, using the same parameters as CEST. CEST maps were computed using the equation CESTasym (%) = 100×[(S-ve – S+ve)/S0], where S-ve, S+ve, and S0 are the B0 corrected MR signals acquired while saturating at –1 ppm, +1 ppm, and 20 ppm from water resonance, respectively. The CEST contrast map was further corrected for any B1 inhomogeneity. Following the acquisition of the baseline CEST, maltitol (1 M, 1.5 ml) was administered via tail vein at a bolus variable rate using a syringe pump for 60 min. Immediately following the beginning of the infusion of maltitol, the CEST acquisitions were performed for 100 minutes. For control studies, rats without any tumor were used and CEST maps were acquired pre-, during and post-infusion of maltitol.Results and Discussion
High-resolution 1H NMR spectra of maltitol solution at low temperature showed the resonances from –OH groups at 0.8 and 1.3 ppm downfield of water due to the slower exchange with water (Fig. 1a). The CEST studies on maltitol phantom showed a broad CESTasym peaking at 1 ppm (Fig. 1b). The malCEST was found to correlate positively with the concentration (Fig. 1c) and negatively with the pH (Fig. 1d). With the saturation pulse parameters used in the current study, ~3% CEST contrast was observed at 1 ppm in the healthy rat brain. This could be due to the endogenous –OH groups predominantly on m-inositol6 and glucose1,2. The CEST contrast in the normal rat brain did not change following the administration of maltitol suggesting that intact blood-brain barrier (BBB) is impervious to maltitol (Fig 2, Upper panel). Increased malCEST contrast was observed in the tumor region at ~30 min during the infusion of maltitol in the rat glioma model (Fig. 2, Middle panel). In the normal-appearing brain region of these rats, malCEST contrast did not change appreciably over the course of CEST acquisition, again indicating that maltitol did not get into the healthy regions of the brain. The malCEST maps provide both visual and quantitative detection of tumor and comparable contrast to T2 weighted and Gd-enhanced images in the tumor region (Fig 2 Lower Panel). Additionally, the z-spectra acquired pre and post administration of maltitol exhibits the elevation of CEST at 1 ppm suggesting that the contrast was provided by the maltitol accumulated in the tumor region (Fig. 3 a, b). Overall, this inexpensive, non-nutritive sweetener in conjunction with its CEST properties can be readily used for examination of various tumors on ultra-high field MRI scanners. This preliminary study paves the way for the development of maltitol and other maltitol derivatives as MRI contrast agents for a variety of preclinical imaging applications as well as to monitor therapeutic response.Acknowledgements
This project was supported by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health through Grant Number P41-EB015893 and the National Institute of Neurological Disorders and Stroke through Award Number R01NS087516 and a grant from Sidra Medical and Research Center.References
1. Walker-Samuel S et al. Nature Med (2013) 19:1067-72; 2. Chan KW et al. Magn Res Med (2012) 68:1764-73; 3. Rivlin M et al. Magn Res Med (2014) 72:1375-80; 4. Xu X et al. Tomography (2015) 1:105-14; 5. Wang J et al. Sci Rep (2016) 6:30618; 6. Haris M et al. Neuroimage (2011) 54:2079-85Figures
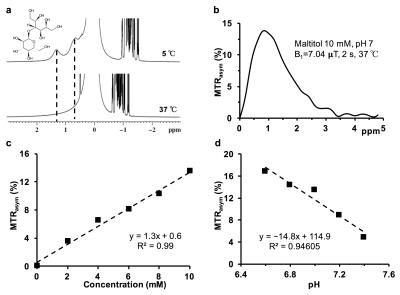
CEST effect from maltitol a) Chemical structure of maltitol showing the exchangeable hydroxyl groups and the high-resolution NMR spectrum of 300 mM maltitol solution showing two peaks from the hydroxyl protons at 0.8 and 1.3 ppm at 5 ºC due to slower exchange b) asymmetry plot from 10 mM of sucralose (pH 7, 37 ºC) shows a broad resonance which peaks at 1 ppm c) the graph shows a linear increase in the sucCEST contrast with the increase in sucralose concentration d) the graph shows a linear reduction in sucCEST contrast with increase in pH.
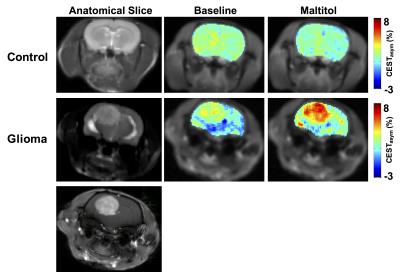
CEST imaging in the rat brain. Upper panel shows the anatomical image of the 4 mm thick coronal brain slice in a control rat brain, malCEST contrast map using parameters (B1 2.2 μT, duration 2s) from the slice prior to the infusion of maltitol and the malCEST contrast map showing no appreciable change following the administration of maltitol. Middle panel (from left to right) shows the anatomical slice, pre- and post-maltitol administration malCEST maps in rat with glioma showing higher CEST in the tumor region compared to the unaffected region. Lower panel showing the Gd-enhanced image of the glioma.
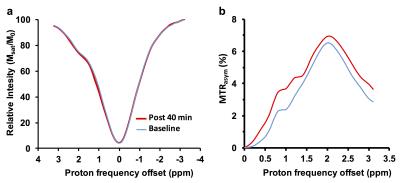
Z-spectra and asymmetry plots in the rat brain. a) z-spectra from the tumor region in the glioma pre- and post-administration of maltitol, b) asymmetry plots showing the increase in CEST contrast at 0.8 and 1.3 ppm following the maltitol administration.