3573
A novel multimodal mannan-based polymer system suitable for tumor and metastasis diagnosisAndrea Galisova1, Marketa Jiratova1, Maria Rabyk2, Martin Hruby2, Milan Hajek1, and Daniel Jirak1
1MR Unit, Institute for Clinical and Experimental Medicine, Prague, Czech Republic, 2Institute of Macromolecular Chemistry, Prague, Czech Republic
Synopsis
Presented mannan-based polymers have promising properties for tumor and metastasis imaging due to their biocompatibility, nanosize and specificity for the immune cells. In this study, two mannan-based polymers were tested by multimodal imaging (MRI and fluorescence). The polymers showed superior imaging properties compared to a commercially available contrast agent. The polymer modified with oxazoline expressed slower elimination rate from the body. Both probes were visualized by MR and optical imaging modality at the injection sites and in the lymph nodes of the experimental mice suggesting their promising properties for cancer diagnosis.
Introduction
Mannan is a biocompatible and biodegradable polysaccharide with a potential for incorporation of drugs and it can be prepared in the form of nanopolymers allowing its use as a drug delivery system with tumor-targeting properties. Moreover, mannan is preferably uptaken in the macrophages and dendritic cells1; what could be useful in diagnosis of the sentinel lymph nodes and metastasis. In this study, mannan polymers were modified with oxazoline for slowing-down of the elimination rate from the body. A gadolinium chelate and a fluorescent near-infrared dye Dy-800 were bounded to the agent for multimodal imaging. The mannan-based probes with (MN-Ox) or without oxazoline (MN) were characterized and compared with a commercially available contrast agent gadoterate meglumine (GM) by MR and fluorescence imaging (FLI). In vivo biodistribution and accumulation of the probes was examined in animals.Material and Methods
Mannan was prepared by allylation with allyl bromide and cysteamine was added by thiol-click addition; then DOTA-Gd(III) complex, and a fluorescent dye Dy-800 were added via the reaction with the corresponding succinimidyl esters and Gd+ chelation. The MR properties of the probes were assessed by r1 (0.5T, saturation recovery sequence, recycle delay 12 s or 5 s) and r2 relaxometry (0.5T, CPMG sequence, recycle delay 10 s, interpulse delay 1 ms, 5000 points), MR imaging (4.7T, T1-weighted images, turbo spin echo sequence, TR=125 ms, TE=11ms, TF=2, resolution 0.27x0.27x1.5 mm3, scan time = 6.5 min) and fluorescence imaging (10 s exposure, excitation 745 nm, emission 810-875 nm). Contrast-to-noise ratios (CNR) from MR images and average fluorescence radiance from FLI images were calculated for each probe concentration in the phantoms. For in vivo monitoring, 150 µL of MN and MN-Ox was administered into the calf muscle of the right hind leg of two B6 healthy mice (the left leg served as control). The agents were dissolved in saline solution reaching the same concentration of a fluorescent dye - 40 µg/mL; the concentration of Gd3+ was 0.4 mg/mL for MN and 4.9 mg/mL for MN-Ox. T1-weighted MR images of the animals were acquired by a turbo spin echo sequence with the parameters: axial images TR=280 ms (7 slices), coronal images TR=336 ms (11 slices), TE=12 ms, TF=2, resolution 0.14x0.14x0.70 mm3, scan time = 7 min. In vivo fluorescent images were acquired at various time points (0-48 hours) after the agent injection using the same adjustments as for the phantoms.Results
Both MN-based agents have higher r1 and r2 relaxivites than GM (Fig. 1A,B); relaxivites of MN and MN-Ox were comparable. Similarly, MR signal and corresponding CNR values of MN-based probes were higher compared to GM (Fig. 1C, D). Mannan agents showed a strong fluorescence signal; fluorescence of GM was in the range of background (Fig. 1E, F). After intramuscular administration in the animals, both MN and MN-Ox agents were visualized at the injection sites by MRI and FLI for 48 hours. FLI signal increased and stayed stable between 4 and 19 hours after injection; then FLI signal of MN slowly decreased, while MN-Ox fluorescence stayed high (Fig. 2A,B). A strong fluorescence signal was detected also from the lymph nodes with higher signal in the case of MN (Fig. 2D). Moreover, FLI signal was visualized at the liver site 8 hours after probes injection with the higher FLI signal originated from MN (Fig. 2C). MR imaging confirmed the presence of the injected probes at the muscle sites (Fig. 3A,B) and in the lymph nodes of both mice (Fig. 3C).Discussion
The novel mannan-based compound possesses superior properties compared to the commercially available contrast agent gadoterate meglumine including MR relaxivity and possibility of fluorescence imaging. The mannan-based nanoprobes were presented at the injection sites for 48 hours indicating slow biodegradation. The appearance of lower FLI signal at the liver and higher signal at the injection site after 48 hours in the mouse with MN-Ox suggests slower elimination process due to addition of oxazoline. Accumulation of the agents detected in the lymph nodes confirmed its immune-targeted property.Conclusion
Here, we presented a novel multimodal mannan-based probe which possesses sufficient sensitivity for fluorescence imaging and superior MR properties compared to a commercial contrast agent. Easily modified chemical structure for drug incorporation, suitable size for enhanced accumulation in tumors and specificity for lymph nodes make this agent promising as a drug delivery system targeted to the tumor tissue and metastasis.Acknowledgements
Supported by MH CR-DRO (Institute for Clinical and Experimental Medicine IKEM, IN00023001) and the Ministry of Health, Czech Republic (grant #15-25781A).References
[1] Cui Z. et al. Drug Dev Ind Pharm, 29(6), 2003Figures
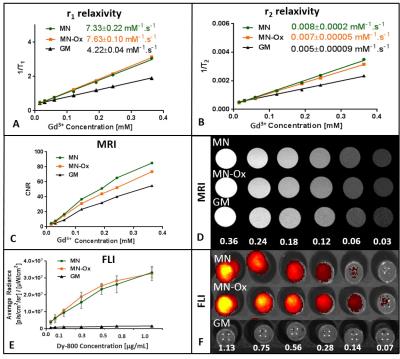
Fig.1 Characterization
of the probes. 1H r1 (A) and r2
(B) relaxivites of the tested probes measured at 0.5T (25⁰C). CNR were calculated from MR images as a contrast between the agent and a water
reference (C). MR images of the probes with different Gd3+ concentration (D); the numbers represent Gd3+ concentration expressed in mM. Average radiance was measured from the area covering one well
with each agent concentration (E). FLI images of the probes with different dye concentration (F); the numbers represent concentration of a fluorescent dye expressed in µg/mL.
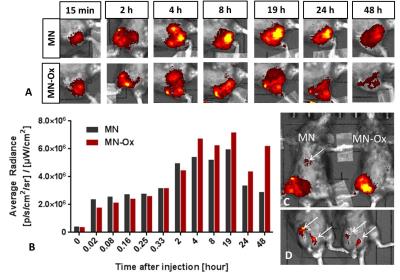
Fig.2 In vivo fluorescence imaging.
The fluorescent images of the right mouse leg with the injected
mannan-based probes at various time points after injection (A). The
quantitative analysis of the fluorescence originating from the muscle of the
mice in time (B). Accumulation of the MN probe in the liver after 19 hours (C)
and in the lymph nodes after 48 hours is pointed by the arrows (D).
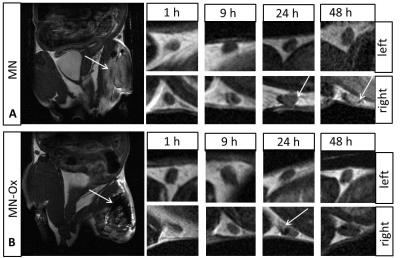
Fig.3 In vivo MR images. Representative
MR images of the mice with injected MN (A) and MN-Ox (B) into the muscle
(pointed by an arrow). The small images show the lymph nodes at various time
points after probe injection. The arrows point hyperintense signal due to
accumulation of the agents.