3572
Direct hyperpolarization of micro- and nanodiamonds for bioimaging applications – considerations on particle size, functionalization and polarization loss1Institute for Biomedical Engineering, ETH Zurich, Zurich, Switzerland, 2Physical Chemistry, ETH Zurich, Zurich, Switzerland
Synopsis
Due to the inherently long relaxation time of 13C spins in diamonds, the nuclear polarization enhancement obtained with dynamic nuclear polarization can be preserved for a time on the order of ~1h, opening up a window for a new class of long-lived contrast agents. Up to now, no imaging with hyperpolarized micro and nanodiamonds has been reported. The present communication presents the feasibility of applying directly hyperpolarized diamonds in MR imaging including considerations for potential in-vivo applications.
Introduction
In dynamic nuclear polarization (DNP), the large nuclear polarization is built by transferring the spin order from an adjacent electron spin bath1. The time available for imaging applications is intrinsically limited by the T1 of the polarized nuclei. Due to the relatively long relaxation time of 13C nuclei at room temperature, typical DNP probes allow for an acquisition window of 30-70s2, which is sufficient to observe fast metabolic pathway or rapid enzymatic activity. Nonetheless, this time window is still very short compared to other imaging modalities, such as FDG-PET3, which is characterized by a half-life time of ~ 2h.
Recently, a novel hyperpolarized agent has been proposed, based on direct polarization of nano- and micro-diamonds4–6. Due to the inherently long relaxation time of 13C spins in diamonds7 of up to tens of minutes, the polarization can be efficiently stored. In addition, the possibility to create a specific targeting by selective surface functionalization as well as the minor toxicity of diamonds themselves are promising characteristics in view of bioimaging applications.
Methods
Hyperpolarization
All experiments were conducted with micro- and nanodiamonds ( Microdiamant AG, Lengwil, Switzerland) in the form of a polycrystalline powder with an average particle size (APS) varying between 125nm to 10μm. The nuclear polarization of 13C nuclei was enhanced by dynamic nuclear polarization exploiting endogenous defects of bulk nitrogen-vacancy (NV) centres and dangling bonds on the surface of the micro- and nanodiamonds. A home-built polarizer operating at B0=3.4T and temperature of 3.5K was used as described previously8. Samples were composed of 180mg tidily packed powder enclosed in a PTFE cup.
In order to improve biocompatibility of the particles, the surface was functionalized with mPEG polymer as reported previously9.
Imaging
After a sufficient time of continuous polarization (depending on the average size of the powder particles, varying between 30-120min), the samples were taken out of the polarizer and immediately transferred to the face of a horizontal 9.4T imaging system (Bruker BioSpin, Ettlingen, Germany) using a permanent, neodymium, tubular magnet. A dedicated coil system (Rapid Biomedical, Wuerzburg, Germany) based on a volume resonator and a receive-only, half-saddle coil was used. Imaging was performed using Rapid Acquisition with Refocused Echoes (RARE) sequence10, employing 71% partial Fourier sampling of a 64x64 acquisition matrix.
Results
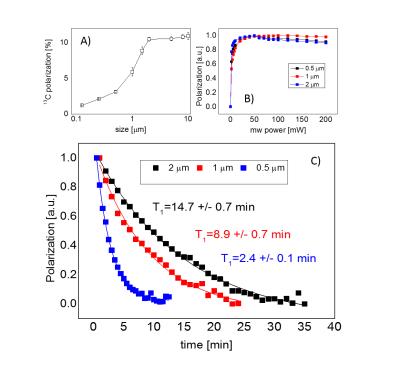
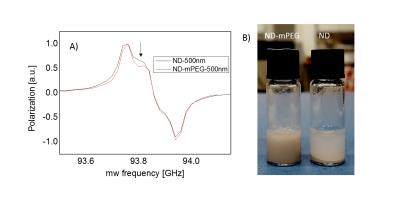
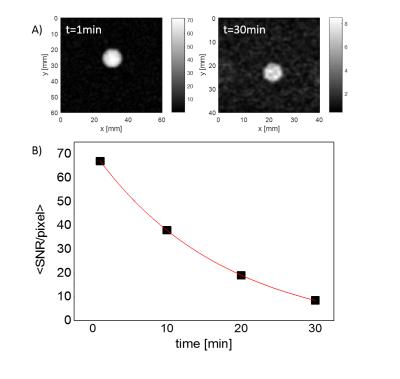
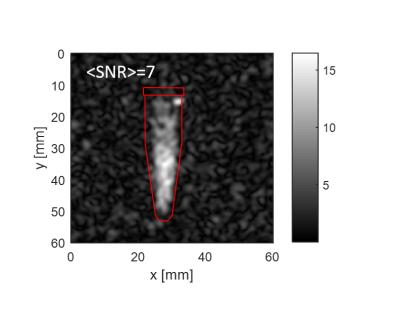
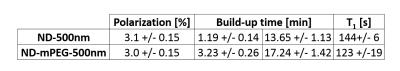
The maximum achievable polarization varied between ~1% for very small particles (125nm) to ~10% for large ones (5-10μm) (Fig.1A). Due to the long electronic relaxation, the microwave power necessary to obtain the maximum polarization was limited to ~60mW and was found to not depend on the particle size (Fig.1B). The room temperature relaxation time inside the imaging system increased with the particle size, from few tens of seconds for small particles to tens of minutes for large ones (Fig.1C). The functionalization with mPEG substantially increased the stability of the particles in an aqueous buffer, whereas bare nanodiamonds did aggregate within ~1min after dispersion (Fig.2B). The functionalization itself did not change polarization nor the room temperature relaxation (Tab.1). A small change in the dangling bonds surface density was observed (Fig.2A, indicated with an arrow) which further resulted in slightly longer build-up time. Good quality images could be obtained up to 30min after transfer of hyperpolarized nanodiamonds in powder form to the imaging system for APS=2μm (Fig.3). When dispersed in an aqueous buffer, image SNR was significantly reduced (Fig.4.). In both experiments, a large loss of polarization upon the transfer from the polarizer’s magnet was observed (on average, 85% of the initial polarization was lost).Discussion
In line with previously published studies5,6,11, this work has demonstrated that micro- and nanodiamonds powder can be efficiently polarized, with maximum achievable polarization of ~10% for 5μm diamonds. The room temperature relaxation was found to be on the order of up to tens of minutes for large diamond particles, facilitating storage of 13C polarization and subsequent imaging at up to 30min after polarization. Surface modification necessary for an in vivo application did not result in a significant change in any of the physical parameters. However, the main obstacle for a potential application of hyperpolarized diamonds is the large loss of polarization upon escape from the polarizing magnet, probably, due to crossing of one of the NV centre’s electron-nucleus spin transition12 that results in a very rapid 13C relaxation (~1s). Further improvement in the sample transfer is necessary to overcome this problem. In addition, in-vivo application of micrometre size particles can be difficult due their inherent aggregation at the side of an injection. Investigation of selective modification of diamonds surface is necessary to help optimizing the polarizability of small particles.Acknowledgements
No acknowledgement found.References
1. Köckenberger, W. Dissolution Dynamic Nuclear Polarization. eMagRes 3, 161–170 (2014).
2. Keshari, K. R. & Wilson, D. M. Chemistry and biochemistry of 13C hyperpolarized magnetic resonance using dynamic nuclear polarization. Chemical Society reviews 43, (2014).
3. Elsinga, P. H. Present and future of PET-radiopharmaceuticals. Nucl. Med. Rev. 15, 13–16 (2012).
4. Dutta, P., Martinez, G. V. & Gillies, R. J. Nanodiamond as a new hyperpolarizing agent and its 13C MRS. J. Phys. Chem. Lett. 5, 597–600 (2014).
5. Rej, E., Gaebel, T., Boele, T., Waddington, D. E. J. & Reilly, D. J. Hyperpolarized nanodiamond with long spin-relaxation times. Nat. Commun. 6, 8459 (2015).
6. Bretschneider, C. O. et al. On The Potential of Dynamic Nuclear Polarization Enhanced Diamonds in Solid-State and Dissolution 13CNMR Spectroscopy. ChemPhysChem 1–12 (2016). doi:10.1002/cphc.201600301
7. Reynhardt, E. C. & Terblanche, C. J. 13C relaxation in natural diamond. Chem. Phys. Lett. 269, 464–468 (1997).
8. Batel, M. et al. A multi-sample 94 GHz dissolution dynamic-nuclear-polarization system. J. Magn. Reson. 214, 166–174 (2012).
9. Wang, D. et al. PEGylated nanodiamond for chemotherapeutic drug delivery. Diam. Relat. Mater. 36, 26–34 (2013).
10. Nitz, W. R. Fast and ultrafast non-echo-planar MR imaging techniques. Eur. Radiol. 12, 2866–2882 (2002).
11. Casabianca, L. B., Shames, A. I., Panich, A. M., Shenderova, O. & Frydman, L. Factors affecting DNP NMR in polycrystalline diamond samples. J. Phys. Chem. C 115, 19041–19048 (2011).
12. Shames, A. I. et al. XRD, NMR, and EPR study of polycrystalline micro- and nano-diamonds prepared by a shock wave compression method. Phys. Status Solidi Appl. Mater. Sci. 212, 2400–2409 (2015).
Figures