3571
Engineered Protein-Iron Oxide Hybrid Biomaterial for MRI-monitoring of Drug Delivery1Biomedical Engineering, SUNY Downstate Medical Center, Brooklyn, NY, United States, 2Radiology, New York University School of Medicine, New York, NY, United States, 3Chemical and Biomolecular Engineering, New York University Tandon School of Engineering, Brooklyn, NY, United States, 4Chemistry, New York University, New York, NY, United States
Synopsis
Our goal is to create protein engineered biomaterials for dual drug delivery and MRI monitoring to improve the growing field of theranostics. Here we present a drug binding protein that is templated to USPIOs using a biomineralization-inspired biotemplation method. The agent shows promise for T2/T2*-weighted MRI.
Introduction
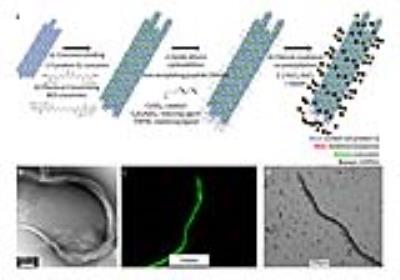
In the growing field of theranostics, combining imaging with drug therapy, image-ready multifunctional biomaterials could greatly advance the way we treat diseases including cancer. Our work aims at coupling MRI and protein engineering to generate trackable therapeutic platforms rationally designed on the genetic level with controlled assembly. Our therapeutic system is based on a coiled-coil protein Q, maintaining a hydrophobic pore for binding small molecules, such as anti-neoplastic curcumin. Q has been engineered to maintain charged surface patches for nanofiber assembly and, upon curcumin binding, fluorescent micron-scale constructs1. For diagnostic purposes, we exploited ultrasmall superparamagnetic iron oxide particles (USPIOs) due to their high sensitivity and tolerance in vivo. USPIO synthesis, however, often consists of harsh conditions followed by surface coating and additional functionalization. To avoid these methods, we take advantage of a CMms6 peptide, derived from the C-terminus of the iron templating protein Mms6 of magnetotactic bacteria2. Mms6 binds and organizes iron oxide (magnetite) particles, without subsequent surface alterations. Using azide-alkyne functionalization, an alkyne-functionalized CMms6 is covalently conjugated to an azide-functionalized variant of Q. By linking curcumin-carrying Q to CMms6, we have created a drug carrying scaffold capable of templating and organizing USPIOs called USPIOH, for USPIO-hybrid biomaterial, for small molecule delivery monitored via T2/T2*-weighted MRI.Methods
Synthesis: Wild type and azide-functionalized Q were expressed in methionine auxotrophic E. coli M15MA. Using residue-specific incorporation of non-natural amino acid azidohomoalanine, azide-functionalized Q was produced3. Purified proteins were dialyzed into acidic buffer initiating nanofiber assembly and subsequently bound to curcumin1, forming drug-carrying micron-scale constructs then chemically crosslinked for stability. Crosslinked constructs were covalently attached to an alkyne-funtionalized CMms6 via azide-alkyne cycloaddition4 to link Q to USPIOs, forming USPIOH (Figure 1a). USPIOs were synthesized via room temperature (RT) co-precipitation of FeCl3 and FeCl2 by NaOH in the absence or presence of CMms6, Q-CMms6, or wild type Q incapable of conjugating CMms6. For validation, our protein-mediated USPIO synthesis was compared to a traditional high temperature reaction in which iron oxide was precipitated and mixed at 85°C5.
Characterization: Transmission electron microscopy (TEM, JEM-1400, JEOL) assessed protein nanofiber assembly and USPIO organization. Electron diffraction rings allowed for the calculation of USPIO d-spacing to confirm magnetite composition. Fluorescence and bright-field microscopy (DMI4000 B, Leica) was used to assess micron-scale protein constructs.
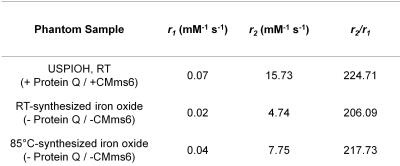
MRI: MRI sequences were evaluated on a 7T Bruker micro-MRI using degassed 2% agarose phantoms containing known amounts of iron oxide samples, including clinically-used Feraheme for comparison. Relaxivity values were assessed using 2D relaxation maps and mono-exponential fitting of the time points acquired. To quantify T1, T2, and T2* times, we used a Look-locker sequence with TE=8.92ms-12.608s, a multi-slice multi-echo sequence with TE=11.034ms-706.176ms, and a multi-gradient echo sequence with TE=2.8ms-60.25ms, respectively. A T1-weighted image was also acquired at TR=150ms to visualize the samples’ T1 effect.
Results
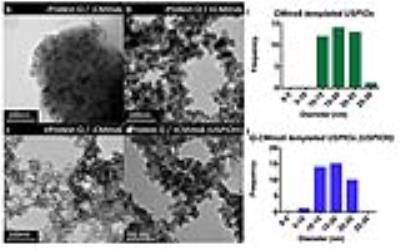
TEM of azide-functionalized Q prior to curcumin-binding revealed nanofibers averaging 128.2nm ±137.7nm, N=33 (Figure 1b). Following curcumin-binding and crosslinking, fluorescence microscopy showed micron-scale assembly averaging 25.7µm ±12.7µm diameter, N=72 (Figure 1c). After cycloaddition to CMms6 and USPIO templation, bright field microscopy of USPIOH suggested that mesofibers were coated in USPIOs (Figure 1d). TEM of iron oxide confirmed that in the absence of CMms6, USPIOs are not formed at RT (Figure 2b,d). However, CMms6 organized USPIOs (Figure 2b) with a diameter of 18.01nm ±4.05nm, N=40 (Figure 2c). Q-CMms6 organizes USPIOs (USPIOH) averaging 16.88nm ±3.74nm, N=40 (Figure 2e). Diffraction rings of USPIOs templated by CMms6 and Q-CMms6 (Figures 3a and 3b) revealed d-spacing in agreement with that of well-described magnetite6, confirming their magnetite composition. T1, T2, and T2*-weighted MRI (Figure 4) showed that RT iron oxide in the absence of CMms6 causes minimal signal change in all three sequences, perhaps due to its lack of organization as demonstrated on TEM (Figure 2a). While all house-made constructs showed minimal T1 effect, Q-CMms6-templated USPIOH sample performed better as a T2/T2* agent than iron oxide synthesized in the absence of protein at RT and 85°C, and it maintained a 7.4-fold higher r2/r1 than Feraheme (Figures4 and 5).Discussion
By biologically synthesizing a coiled-coil protein and utilizing bio-inspired iron oxide templation, we engineered a trackable USPIO-functionalized drug-carrying hybrid scaffold, USPIOH. TEM studies confirmed that its ability to organize USPIOs is due to conjugation to iron-templating CMms6 and MRI studies revealed that organized USPIOs are necessary for appreciable relaxation effect. Our USPIOH was validated by its higher relaxivities than the well-established high temperature USPIO synthesis method and its 7.4-fold higher r2/r1 than Feraheme, suggesting its use as a theranostic agent enabling chemotherapeutic delivery to be visualized via T2/T2*-weighted MRI.Acknowledgements
This work was supported by ARO (W911NF-11-1-0449) and NSF MRSECProgram award number DMR-0820341.References
1 Hume J, et al. Biomacromolecules. 2014; 15: 3503-10.
2 Arakaki A, et al. J Biol Chem. 2003; 278: 8745-50.
3 Link AJ and Tirrell DA. J Am Chem Soc. 2005; 125 (37): 11164-5.
4 Hong Vu, et al. Angew Chem Int Ed. 209; 48: 9879-83.
5 Merida F, et al. J Mag and Mag Mat. 2015; 394: 361-71.
6 JCPDS- International Centre for Diffraction Data. 1986. Power Diffraction. Swathmore, PA: JCPDS- International Centre for Diffraction Data.
Figures