3570
3 nm iron oxide nanoparticles as spin-lattice relaxation time enhancing contrast agents.1Neurology, Washington University School of Medicine in St. Louis, St. Louis, MO, United States, 2Seoul National University, 3Washington University School of Medicine in St. Louis
Synopsis
Relaxation based MR images have superior spatial resolution compared to other MRI approaches. In addition, structural MRI requires short scan time and yet has high signal to noise ratio. However, the greatest limitation of structural MRI is poor specificity and sensitivity to pathology. Thus a well characterized MR molecular contrast agent would be a major advance. Here we present 3 nm iron oxide nanoparticles (IONP) as a T1 contrast agent. The 3 nm IONP showed strong T1 effects up to 13.5 ms echo time. A fully functionalized 3 nm IONP would be an appropriate component for molecular contrast agents.
Purpose
In the presented study, we examined spin-lattice and spin-spin relaxation enhancing effect (or so called T1 and T2 shortening effect) of iron oxide nanoparticles (IONP). In general IONP has strong spin-spin relaxation time shortening effect due to its strong magnetic dipole moment. The T2 shortening effect significantly increases with the size of IONP (1). However, this causes a major problem for IONP as MRI contrast agents for concussive traumatic brain injury (TBI) and other disorders with intact blood brain barrier. Larger IONPs have highly limited penetration through the blood brain barrier. The small amount of IONP in brain requires heavy T2 weighting to enhance contrast. Yet the heavy T2 weighting also brings up image contrast due to the nature of tissue itself, which might ruin the sensitivity of IONP to specific pathology. Furthermore, larger IONPs can have T2* effects which can be difficult to distinguish from micro hemorrhages. So a well characterized spin-lattice relaxation time enhancing MR contrast agent, which also has small size and can be functionalized by specific binding domains such as antibodies, would be highly beneficial for concussive TBI and many other applications. Recently there are reports that extremely small diameter IONPs have T1 shortening effects (2). Here we present 3 nm IONPs as a candidate of T1 shortening MR contrast agent.Methods
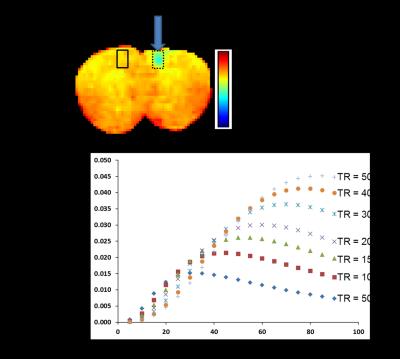
3 nm Iron oxide nanoparticles (IONP) were obtained from our collaborator, Dr. Hyeon’s group who published methods for fabricating extremely small iron oxide nanoparticle (3). The 3 nm IONP was stored in pure water and diluted from 0.5 to 0.02 mM [Fe]. All MRI measurements were performed in Agilent 4.7 T magnet with 60 g/cm gradient. A house-built cylindrical shape radio frequency coils having 2 – 4 cm diameter were used. The T1 (s) (or r1 (s-1)) of 3 nm IONP was measured using an inversion recovery pulse sequence with repetition time (TR, 6 s), echo time (TE, 5 ms), inversion recovery time from 0.01 to 4 s. The T2 (s) (or r2 (s-1)) of 3 nm IONP was measured using Carr Purcell Meiboom Gill sequence with repetition time (TR, 6 s), echo time (TE, 8 ms), and 16 echo train. The r1 and r2 was calculated using Bayesian analysis tool box (http://bayesiananalysis.wustl.edu/). For in situ experiments, 1 µl of 3 nm IONP at iron concentration of 0.1 mM [Fe] was injected into mouse brain right hemisphere. Animals were sacrificed immediately after injection without perfusion then the head underwent drop fixation in 10 % neutrally buffered formalin. The T1 (or r1) map of IONP injected mouse brain was obtained using same image parameters as IONP phantom study. The T1 shortening effect was examined in gradient echo (TE = 3.2 ms, TR = 50, 100 – 500 ms in 100 ms step) and spin echo (TE = 13. 5 ms, TR = 0.1 – 1.5 in 0.1 s step) sequence. All images have 118 x 118 x 500 µm3 voxel size.Results
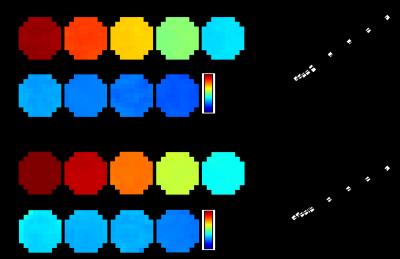
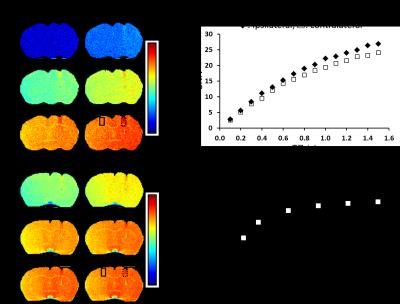
Figure 1 shows r1 and r2 measure of 3 nm IONP at various iron concentrations. The r2/r1 ratio is about 7.9. This clearly shows that the employed 3 nm IONP has strong spin-lattice relaxation enhancing effect. Figure 2 shows T1 map of IONP injected mouse brain and the simulated MR signal difference between ipsilateral and contralateral using Ernst Angle (4). The T1 shortening effect of 3 nm IONP is clearly seen in T1 map (Fig. 2a). The simulation suggests detectable signal difference between ipsilateral and contralateral as short at 50 ms TR. The T1 weighted images were collected using gradient echo and spin echo sequence, Fig. 3. The T1 shortening effect of 3 nm IONP is clearly seen even on spin echo which has relatively long echo time, 13.5 ms, Fig. 3 a and b. The results above clearly show that 3 nm IONP has MR detectable spin-lattice relaxation time shortening effect. The T1 shortening effect is more prominent in gradient echo imaging having short echo time (Fig. 4).Discussion and Conclusion
The results clearly show that 3 nm IONPs have potential to be used as T1 MR contrast agents. Considering their size, the 3 nm IONPs would likely have improved blood brain barrier penetration rate compared to larger particles. In the future, functionalizing 3 nm IONPs with antibody and in vivo application to animal models of concussive TBI model will be performed.Acknowledgements
This study was supported by. NIH U01 NS086659-01, the US Department of Defense, and HealthSouthReferences
1. Shapiro EM. Biodegradable, polymer encapsulated, metal oxide particles for MRI-based cell tracking. Magnetic resonance in medicine 2015;73(1):376-389.
2. Peng Y-K, Tsang SCE, Chou P-T. Chemical design of nanoprobes for T1-weighted magnetic resonance imaging. Materials today 2016;19(6):336-348.
3. Kim BH, Lee N, Kim H, An K, Park YI, Choi Y, Shin K, Lee Y, Kwon SG, Na HB, Park JG, Ahn TY, Kim YW, Moon WK, Choi SH, Hyeon T. Large-scale synthesis of uniform and extremely small-sized iron oxide nanoparticles for high-resolution T1 magnetic resonance imaging contrast agents. Journal of the American Chemical Society 2011;133(32):12624-12631.
4. Ernst RR, Anderson WA. Application of Fourier Transform Spectroscopy to Magnetic Resonance. Review of Scientific Instruments 1966;19(1):93-102.
Figures