3569
Comparison of USPIO and MPIO for molecular MRI across different field strengths1Department of Oncology, University of Oxford, Oxford, United Kingdom
Synopsis
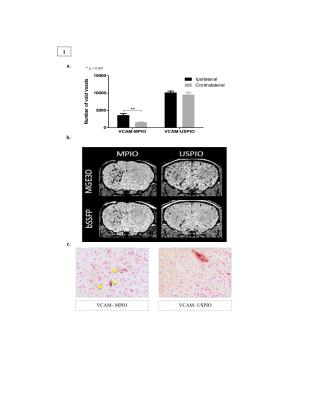
We have previously reported molecular MRI studies using Microparticles of Iron Oxide (MPIO) coupled to anti-VCAM-1 antibodies. However, Ultrasmall Superparamagnetic Iron Oxide (USPIO) are more commonly used in MRI applications. Although MPIO provide greater targeting valency and contrast per particle, their advantages over UPSIO for molecular MRI has not been demonstrated empirically. Here, we compared the sensitivity and specificity of VCAM-MPIO and VCAM-USPIO for molecular MRI; VCAM-MPIO, but not VCAM-USPIO, yielded marked and selective contrast in areas of cerebral inflammation. These results confirm the theoretical assumption that MPIO present a more viable platform for molecular MRI than USPIO.
PURPOSE
Molecular Magnetic Resonance Imaging (mMRI) in preclinical in vivo models is an active area of research with the overall goal of visualising specific molecular biomarkers involved in various diseases. Superparamagnetic contrastophores have stimulated a variety of applications in this field. These particles create potent hypointensities on MR images acquired by sequences that are sensitive to local inhomogeneities of the magnetic field. It has been shown that mMRI can be performed using Micro Particles of Iron Oxide (MPIO, ~ 1um) coupled to antibodies targeting Vascular Cell Adhesion Molecule 1 (VCAM-1)1. However, Ultrasmall Superparamagnetic Iron Oxide (USPIO, ~ 25 nm) are more commonly used for other MRI applications, such as cell tracking. Although, theoretically, MPIO should provide a more advantageous platform for molecular imaging, owing to greater targeting valency and contrast per particle, this has never been demonstrated empirically. The aim of this study, therefore, was to test the sensitivity and specificity of VCAM-MPIO compared to VCAM-USPIO for the detection of endothelial activation using a mouse model of acute central inflammation. The comparison was made across a range of magnetic fields and with the use of two different sequences previously used for iron oxide detection: balanced Steady-State Free Precession (bSSFP3D) and Multi Gradient-Echo (MGE3D).METHODS
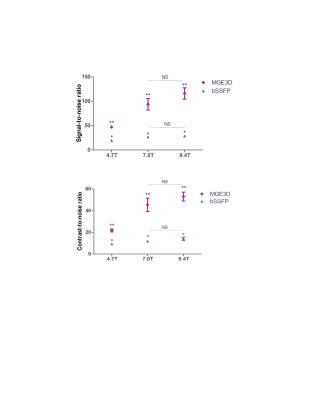
Acute brain inflammation was induced by unilateral stereotactic injection of interleukin 1β (IL-1β) into the left striatum of mice. Animals were injected with either VCAM-MPIO or VCAM-USPIO intravenously three hours after surgery (4mg iron/kg body weight), and then imaged 1 or 13 hours post-injection, respectively. This time allows for specific binding and clearance of unbound particles, based on their respective clearance rates from the bloodstream2. Imaging was performed on 4.7T, 7.0T and 9.4T (Agilent Technologies, USA) preclinical MR scanners. Each subject was imaged with both bSSFP3D (TR=8ms, TE =4ms, flip=20°, Texp=40min) and MGE3D (TR=65ms, TE/TE2=2.5/4.0ms, NE=15, flip=15°, Texp=40min) sequences. In order to eliminate the off-resonance banding artefact of bSSFP3D, multiple SSFP transients with acquisition frequency offsets were used3. Both sequences were reconstructed using sum-of-square algorithm4, for bSSFP3D transients were added, whereas for MGE3D subsequent echoes were summed. For analysis, brains were segmented and the hypointense signal arising from the iron oxide particles were automatically measured by in-house made MATLAB program. Signal-to-Noise (SNR) and Contrast-to-Noise (CNR) ratios were measured for each sequence and scanner. Mice were perfused after each imaging session, and VCAM-MPIO/USPIO binding to VCAM-1-expressing endothelial cells was assessed histologically.RESULTS
VCAM-MPIO displayed higher sensitivity and specificity for imaging cerebrovascular inflammation compared to VCAM-USPIO. Hypointense foci from VCAM-MPIO were almost exclusively found in the IL-1b-injected hemisphere and, quantitatively, were significantly greater than in the contralateral side (Figure 1a-b). In contrast, no significance difference was found between the two brain hemispheres for VCAM-USPIO induced hypointensities (Figure 1a-b). Immunohistochemically, VCAM-MPIO were found associated with VCAM-1 expressing vessels, but no VCAM-USPIO were detected (Figure 1c). MPIO/USPIO-induced hypointensities were clearly detectable on both MGE3D and bSSFP3D images. However, at the same resolution and scan-time, both SNR and CNR were significantly higher using the MGE3D approach (Figure 2). SNR and CNR for both sequences increased with field strength, as expected, although to a greater extent for MGE3D (Figure 2).DISCUSSION
In this study, we have demonstrated specific binding of VCAM-MPIO to inflamed endothelial cells together with specific contrast on both bSSFP3D and MGE3D images. In contrast, no specific binding of VCAM-USPIO was detected histologically, and the presence of bilateral VCAM-USPIO- induced contrast, even several hours post-injection, suggests that the hypointense signals reflect circulating USPIO in the blood. These results confirm the previously theoretical assumption that MPIO present a much more viable platform for mMRI than USPIO. Although MPIO and USPIO presented as void regions on both MGE3D (T2* weighted) and bSSFP3D (T2/T1 weighted) images, MGE3D yielded higher SNR and CNR for detection.CONCLUSION
Whilst classical imaging techniques fail to reveal cerebrovascular inflammation, the field of molecular imaging has been able to tackle this challenge. Although USPIO-based imaging remains an active field of investigation, in this work, we have demonstrated marked limitations for their use in molecular MRI. In contrast, targeted MPIO imaging offers a potent and sensitive platform for such studies, as previously demonstrated.Acknowledgements
The authors would like to thank Paul Kinchesh and Sean smart (Oxford University) for helping with pulse sequence development. This work was funded by Cancer Research UK (grant number C5255/A15935) and the CRUK/EPSRC Cancer Imaging Centre in Oxford (grant number C5255/A16466).References
1 McAteer MA, Sibson NR, et al. In vivo magnetic resonance imaging of acute brain inflammation using microparticles of iron oxide.Nat. Med. 2007; 13(10): 1253–1258.
2 Melemenidis S, Jefferson A, et al. Molecular Magnetic Resonance Imaging of Angiogenesis In Vivo using Polyvalent Cyclic RGD-Iron Oxide Microparticle Conjugates. Theranostics. 2015; 5(5): 515-529.
3 Ribot EJ, et al. Water Selective Imaging and bSSFP Banding Artifact Correction in Humans and Small Animals at 3T and 7T, Respectively. PLoS One. 2015: 10(10): e0139249.
4 Bangerter NK, et al. Analysis of Multiple-Acquisition SSFP. Magn Reson Med. 2004; 51(5): 1038-47.
Figures