3568
A Transgenic Method to Enhance Intracellular Retention of Iron Oxide Nanoparticles and Prolong MRI Tracking1Bourns college of engineering, University of California, Riverside, CA, United States, 2Department of Biomedical Engineering, Emory University
Synopsis
Iron oxide nanoparticles (IONPs) are widely used as labels for noninvasive cell tracking using magnetic resonance imaging (MRI). However, IONP induced contrast dilutes rapidly over time due to cell growth and division, limiting its applications in long-term cellular visualization. In the present work, unlike techniques focusing on modification of IONPs, we established a novel transgenic approach to increase IONP retention, significantly prolonging the tracking time of IONP labeled cells. In addition, this approach could be generalized to other cell types and magnetic nanoparticles, making it attractive for long-term cell tracking or tumor progression monitoring.
Introduction
Iron oxide nanoparticles (IONPs) have been used widely in noninvasive cell tracking and tumor monitoring because of their favorable biocompatibility and high sensitivity in MRI.1 However, the MR contrast of IONPs labeled cells diminishes rapidly over time due to cell growth and division,2 limiting long term tracking of IONPs labeled cells in vivo. Mms6, a protein identified in magnetotactic bacteria (MTB), has been reported to play a key role in the formation of magnetosomes in MTB.3 We have previously shown that eukaryotic expression of Mms6 can form clusters of nanoparticles in mammalian cells.4 However, whether Mms6 expression in tumor cells could extend the retention of IONPs has not been investigated. In the present study, we established transgenic tumor cells stably expressing Mms6 protein and evaluated their capacities to retain IONPs in a rodent model.
Materials and Methods
Establishment of Mms6-expressing cell lines: A rat glioma cell line, 9L obtained from Dr. Hyunsuk Shim at Emory University, was cultured in Dulbecco’s Modified Eagle’s Medium Medium (DMEM) (ATCC, Manassas, VA, USA) with 10% heat-inactivated fetal bovine serum (Sigma–Aldrich, St. Louis, MO, USA). Wild-type 9L (9L-wt) cells were transfected with pcDNA3.1/nV5-mms6. After geneticin-resistant clones were formed and expanded, western blot and fluorescence imaging assays were performed to ascertain the expression of Mms6 in mms6-positive cells (9L-mms6).
Intracellular iron measurement: 9L-wt and 9L-mms6 cells were incubated with various concentrations of IONPs for 12 h. After labeling with IONPs, 9L-wt or 9L-mms6 cells were cultured in DMEM medium without IONPs for 2 weeks. At each time point (days 1, 4, 7, 10, and 14), cells were collected to quantify iron content using the inductively coupled plasma mass spectroscopy (ICP-MS).
Rodent tumor model: Fischer 344 rats were purchased from Charles-River laboratories (Wilmington, MA, USA). All rats receiving flank tumors were injected on the lateral sides of the two hind legs. Both 9L-wt and 9L-mms6 cells were freshly harvested, sorted and transplanted.
MRI: Both cell imaging and animal imaging were performed on a 9.4 T MR scanner (Bruker, Billerica, MA, USA). T2 and transverse relaxation rate (R2 =1/T2) were calculated by fitting decay curves measured using a Multi-Slice Multi-Echo (MSME) sequence with the following parameters: TR = 4000 ms, TE = 9.3~111.6 ms spaced by 9.3 ms, echoes = 12, FOV = 60 × 60 mm2, matrix size = 128 × 128, and slice thickness = 1 mm.
Results and Discussion
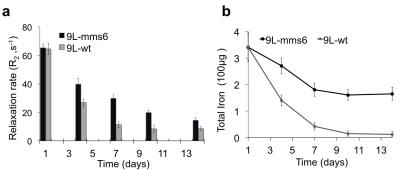
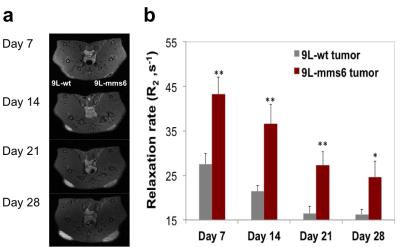
As illustrated in immunofluorescent staining (Figure 1), only 9L-mms6 cells are labeled in red but not in 9L-wt cells, demonstrating that Mms6 is expressed in 9L-mms6 cells but not in wild-type control 9L cells (9L-wt). Figure 2a shows the R2 value related to IONPs concentration at days 4, 7, 10, and 14, confirming that 9L-mms6 cells retained IONPs-induced MR contrast for a much longer time than 9L-wt cells. Due to rapid cell replication, IONP concentrations in both 9L-wt and 9L-mms6 cells decayed over time but with different rates. As shown in Figure 2b, the total iron contents in 9L-mms6 cells did not go down to background level as 9L-wt did over the two-week observation period. Instead, the decay of iron content after day 7 was much slower in 9L-mms6 cells, indicating that Mms6 plays an important role in the retention of IONPs. Figure 3a shows in vivo T2–weighted images in which the image intensity of the 9L-mms6 tumors on the right is significantly darker than that of the 9L-wt tumors on the left at days 7, 14, 21 and 28. Figure 3b plots the R2 values of both tumors at different time points. We found that 9L-wt tumors lost the IONP-induced MR contrast after 2 weeks. Instead, the IONP contrast was still prominent in 9L-mms6 tumors even at 4 weeks after tumor inoculation, indicating that transgenic tumor cells stably expressing Mms6 protein retained IONPs in cells despite rapid cell division for over four weeks, most likely because transgenically produced Mms6 slowed down the exocytosis rate of IONPs. 5
Conclusion
We conclude that the IONPs induced MR contrast could be detected for an extended period of time with the expression of Mms6. Moreover, this approach could be generalized to other cell types and various magnetic nanoparticles, making it attractive for long-term cell tracking in a plethora of biomedical applications.
Acknowledgements
No acknowledgement found.References
1. Barnett BP, et al. Magnetic resonance-guided, real-time targeted delivery and imaging of magnetocapsules immunoprotecting pancreatic islet cells. Nat Med. 2007;13(8):986–991
2. Taylor A, et al. Long-term tracking of cells using inorganic nanoparticles as contrast agents: are we there yet? Chem Soc Rev. 2012; 41(7):2707–2717.
3. Galloway JM, et al. Magnetic bacterial protein Mms6 controls morphology, crystallinity and magnetism of cobalt-doped magnetite nanoparticles in vitro. J Mater Chem. 2011; 21(39):15244.
4. Zhang X-Y, et al. A bacterial gene, mms6, as a new reporter gene for magnetic resonance imaging of mammalian cells. Mol Imaging. 2014; 13:1-14.
5. Xu C, et al. Tracking mesenchymal stem cells with iron oxide nanoparticle loaded poly(lactide-co-glycolide) microparticles. Nano Lett. 2012; 12(8):4131–4139.
Figures