3548
T1- MRI Assessment of Hepatobiliary Fibrosis in Cystic Fibrosis Patients: Significance for Chronic Liver Diseases1Radiology, Case Western Reserve University, Cleveland, OH, United States, 2Medicine, University Hospitals of Cleveland, OH, United States, 3Pediatrics, University Hospitals of Cleveland, Cleveland, OH, United States, 4Urology, Case Western Reserve University, Cleveland, OH, United States, 5Biomedical Engineering, Case Western Reserve University, Cleveland, OH, United States, 6Physiology and Biophysics, Case Western Reserve University, Cleveland, OH, United States, 7Genetics, Case Western Reserve University, Cleveland, OH, United States
Synopsis
Liver disease is the third leading cause of death in Cystic Fibrosis (CF). Unfortunately, conventional liver function tests cannot sensitively detect it.1-3 Liver stiffness measurements via MRI and ultrasound have shown promise but are unfortunately impacted by other factors (e.g., hepatic fat) potentially resulting in over-estimation of fibrosis.4,5 We recently validated a T1-MRI assessment of biliary dilatation and fibrosis in a rat model of congenital hepatic fibrosis (Figs. 1-3).6 In this clinical study, we show that T1-MRI can be used to sensitively detect increased percent bile duct volumes in CF patients in comparison to control subjects with normal liver function.
Purpose
To determine whether T1 relaxation time assessments previously validated in a rat model of congenital hepatic fibrosis could be used to quantitatively assess hepatobiliary fibrosis in cystic fibrosis patients with normal liver function tests.Methods
We recruited six adult cystic fibrosis patients (3 male, 3 female; age=26-62) and five healthy volunteers (2 male, 3 female; age=27-47). The CF patients had a range of lung disease (FEV1 = 38 - 100%) but showed no signs of liver disease as evidenced by elevated liver enzymes (e.g., Gamma-Glutamyl Transpeptidase). The non-CF volunteers had no known liver diseases. All subjects were scanned on a Siemens Skyra 3.0T MRI scanner using a rapid Look Locker MRI acquisition in a single 5-second breath hold (TR/TE = 2.02 ms / 0.97 ms, FOV = 400 mm x 400 mm, matrix size = 64 x 128, flip angle = 7°, slice thickness = 6 mm, 40 images following the initial inversion).7,8 T1 maps were acquired for seven axial slices over each subject’s liver and the images were exported for offline T1 estimation in Matlab using a least-squares fit to an exponential model. A thresholding technique was then used to separate bile ducts (high T1 values) from the normal liver parenchyma (lower T1 values). An ROI analysis was then performed to calculate percent bile duct as: (Bile Duct Volume / Total Liver ROI Volume) * 100%. Large vessels were avoided in order to limit the effects of vasculature in each imaging slice. Each subject’s mean percent bile duct volume was established by averaging over all seven imaging slices. Scatterplots of the mean percent bile duct volume for each subject as well as a two-tailed Student’s t-test were performed to compare the imaging results between the CF patients and the healthy controls. A p-value of less than 0.05 was considered statistically significant.Results
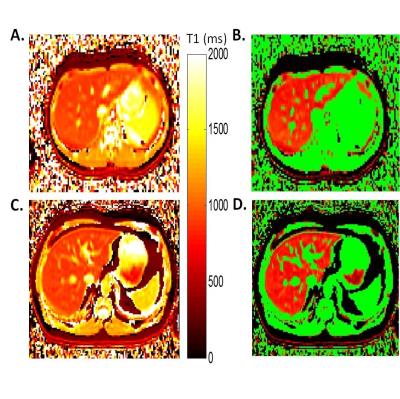
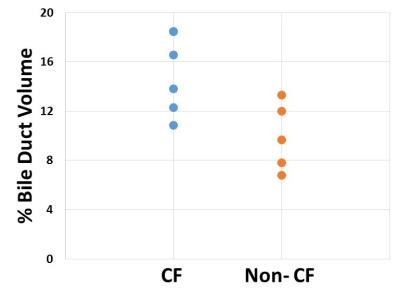
Representative liver T1 relaxation time maps from a CF patient and a non-CF control subject are shown in Figure 4. The CF patients exhibited visible increases in bile ducts (Fig. 4C) similar to the rat model of congenital hepatic fibrosis (Figures 1-3). These T1 maps were then thresholded to distinguish regions of increased T1 values (e.g., bile ducts) from lower T1 values typical of normal liver parenchyma. Pseudo-colored thresholding of the liver T1 maps for the same healthy control subject and CF patient are shown in Figures 4B and 4D. Voxels with T1 values over the threshold are shown in green. The thresholding and subsequent ROI selection (to select the majority of the liver while excluding major blood vessels) were used to calculate the % Bile Duct Volume for each patient and volunteer. Mean values of % Bile Duct Volume for the CF patients were significantly increased relative to the healthy control subjects (15.1% vs. 9.92%, p=0.02) as shown in Figure 5.Discussion
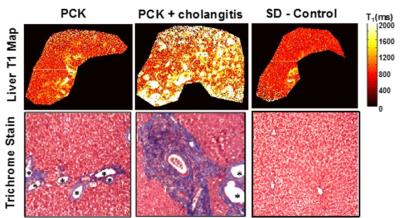
Cystic Fibrosis Liver Disease (CFLD) is the third leading cause of death in CF and exhibits the same pathophysiologic manifestations as the PCK rat: biliary dilatation and associated progressive periportal fibrosis leading to cirrhosis and liver failure.1-3 Importantly, CFLD therapies are also available (e.g. ursodeoxycholic acid, UDCA) aimed at reducing biliary dilatation. Unfortunately, our understanding of CFLD progression as well as the efficacy of liver-specific therapies in individual CF patients is hindered by the lack of a safe and effective test to detect and monitor CFLD. Serum-based liver function tests lack the sensitivity and specificity to detect early-stage liver disease.3 In addition, liver stiffness measurements by elastography techniques are impacted by multiple pathophysiologic features of CFLD including biliary dilatation, fibrosis, steatosis, and/or edema leading to non-specific and potentially erroneous results.4,5 In a previous preclinical study of the PCK rat model of congenital hepatic fibrosis, we showed that T1-MRI assessments: 1) can sensitively detect hepatobiliary dilatation; and 2) were significantly correlated with histologic measures of % bile duct area, % fibrosis area, and collagen content (i.e., hydroxyproline) (Figure 2,3). This is the first clinical evaluation of the capability for T1 relaxometry assessments to sensitively detect hepatobiliary dilatation as an early marker for periportal hepatic fibrosis. In this study, we have shown that CF patients exhibit significantly increased % bile duct volume in comparison to non-CF control subjects (Figures 4,5).Conclusion
Overall, these results suggest that quantitative and rapid T1-MRI assessments (5 seconds / imaging slice) may provide a specific and sensitive assessment of CFLD. Importantly, this straightforward MRI technique may also be relevant for other chronic liver diseases (e.g., Autosomal Recessive Polycystic Kidney Disease, alcoholic liver disease, etc).Acknowledgements
We would like to acknowledge the support of the Cystic Fibrosis Foundation, NIH/NIDDK ROI DK085099, NIH/NIDDK K12 DK100014, the Case Comprehensive Cancer Center (NIH/NCI P30 CA43703), and the Clinical and Translational Science Collaborative of Cleveland (NIH/NCATS UL1 TR000439).References
1. Cystic fibrosis foundation, patient registry 2010 annual data report. Bethesda, md: Cystic Fibrosis foundation; 2011.
2. Davis PB, Drumm M, Konstan MW. Cystic fibrosis. Am J Respir.Crit Care Med. 1996;154:1229-1256
3. Debray D, Kelly D, Houwen R, Strandvik B, Colombo C. Best practice guidance for the diagnosis and management of cystic fibrosis-associated liver disease. J Cyst Fibros. 2011;10 Suppl 2:S29-36.
4. Witters P, De Boeck K, Dupont L, Proesmans M, Vermeulen F, Servaes R, Verslype C, Laleman W, Nevens F, Hoffman I, Cassiman D. Non-invasive liver elastography (fibroscan) for detection of cystic fibrosis-associated liver disease. J Cyst Fibros. 2009;8:392-399.
5. Mueller-Abt PR, Frawley KJ, Greer RM, Lewindon PJ. Comparison of ultrasound and biopsy findings in children with cystic fibrosis related liver disease. J Cyst Fibros. 2008;7:215-221.
6. Gao Y, Erokwu BO, DeSantis DA, Croniger CM, Schur RM, Lu L, Mariappuram J, Dell KM, Flask CA. Initial evaluation of hepatic t1 relaxation time as an imaging marker of liver disease associated with autosomal recessive polycystic kidney disease (arpkd). NMR Biomed. 2016;29:84-89.
7. Dasenbrook EC, Lu L, Donnola S, Weaver DE, Gulani V, Jakob PM, Konstan MW, Flask CA. Normalized t1 magnetic resonance imaging for assessment of regional lung function in adult cystic fibrosis patients--a cross-sectional study. PLoS One. 2013.
8. Donnola SB, Dasenbrook EC, Weaver D, Lu L, Gupta K, Prabhakaran A, Yu X, Chmiel JF, McBennett K, Konstan MW, Drumm ML, Flask CA. Preliminary comparison of normalized t1 and non-contrast perfusion mri assessments of regional lung disease in cystic fibrosis patients. J Cyst Fibros. 2015.
Figures